Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0VEKFU
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
LY-4170156
|
|||||
| Synonyms |
LY-4170156; MBK 103; MBK-103; MBK103
Click to Show/Hide
|
|||||
| Organization |
Mablink Bioscience
|
|||||
| Drug Status |
Phase 1
|
|||||
| Indication |
In total 7 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
8
|
|||||
| Structure |
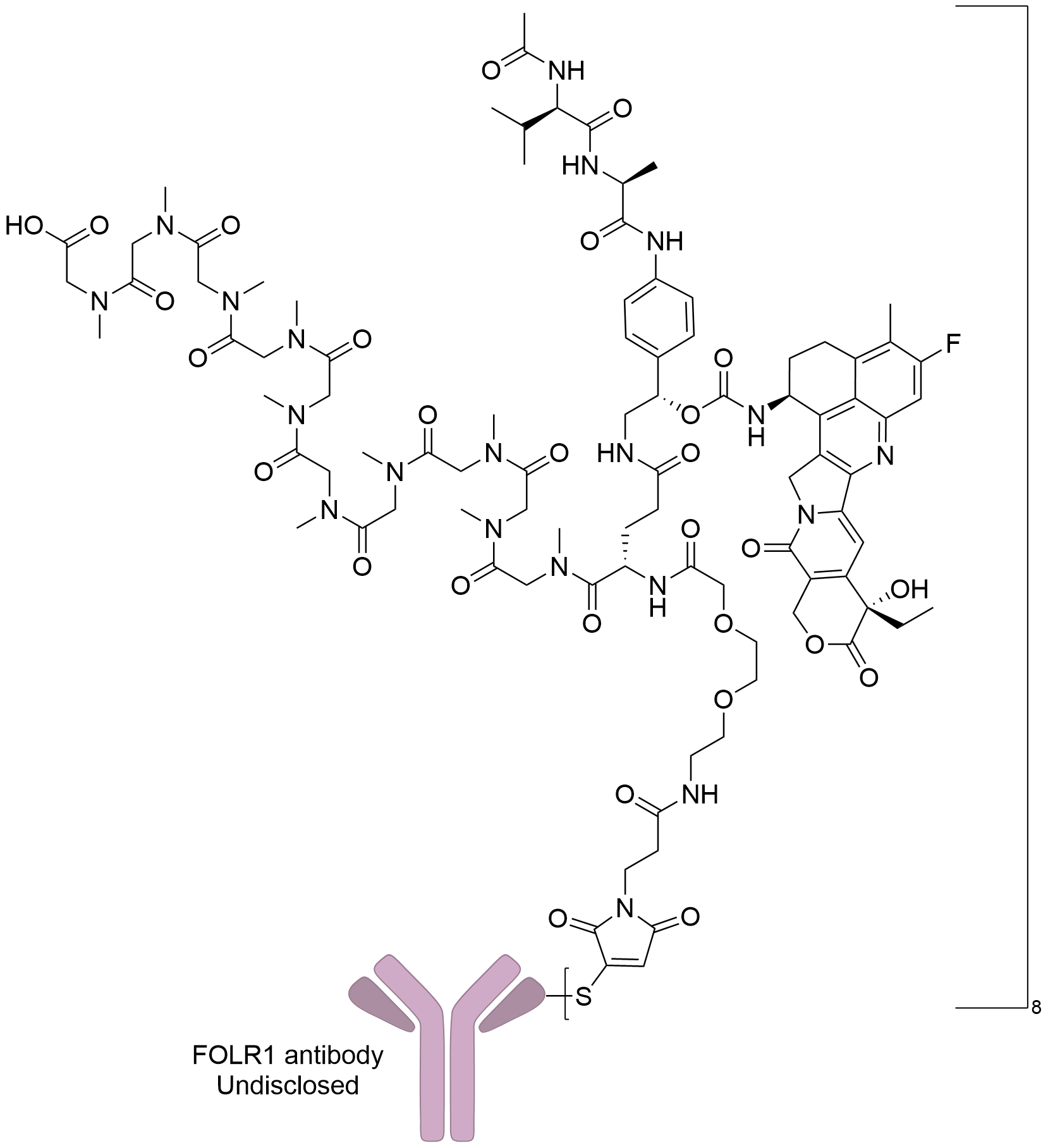
|
|||||
| Antibody Name |
Fc-silent FR specific humanized IgG1 antibody
|
Antibody Info | ||||
| Antigen Name |
Folate receptor alpha (FOLR1)
|
Antigen Info | ||||
| Payload Name |
Exatecan
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mal-Polysarcosine10-Val-Ala-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
