Payload Information
General Information of This Payload
| Payload ID | PAY0LEIGJ |
|||||
|---|---|---|---|---|---|---|
| Name | Exatecan |
|||||
| Synonyms |
Exatecan; 171335-80-1; Exatecan [INN]; Exatecan mesylate; DX-8951; DX-8951f; OC71PP0F89; (1S,9S)-1-Amino-9-ethyl-5-fluoro-1,2,3,9,12,15-hexahydro-9-hydroxy-4-methyl-10H,13H-benzo(de)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-10,13-dione; Dx 8951; UNII-OC71PP0F89; exatecan-mesylate; (1s,9s)-1-amino-9-ethyl-5-fluoro-1,2,3,9,12,15-hexahydro-9-hydroxy-4-methyl-10h,13h-benzo[de]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-10,13-dione; EXATECAN [MI]; EXATECAN [WHO-DD]; SCHEMBL2512959; CHEMBL1614650; DTXSID60169061; CHEBI:135709; EX-A2683; NSC829066; AKOS005146469; AT33978; BCP9000674; DB12185; NSC-829066; 10H,13H-Benzo(de)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-10,13-dione, 1-amino-9-ethyl-5-fluoro-1,2,3,9,12,15-hexahydro-9-hydroxy-4-methyl-, (1S,9S)-; 10H,13H-Benzo(de)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-10,13-dione, 1-amino-9-ethyl-5-fluoro-1,2,3,9,12,15-hexahydro-9-hydroxy-4-methyl-, (1S-trans)-; AC-32495; BP-27995; BP-27996; HY-13631; DB-064817; DX8951;DX 8951;DX-8951; J-521361; Q5419343; (10S,23S)-23-amino-10-ethyl-18-fluoro-10-hydroxy-19-methyl-8-oxa-4,15-diazahexacyclo[14.7.1.02,14.04,13.06,11.020,24]tetracosa-1,6(11),12,14,16,18,20(24)-heptaene-5,9-dione; (1S,9S)-1-amino-9-ethyl-5-fluoro-9-hydroxy-4-methyl-2,3,12,15-tetrahydrobenzo[de]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-10,13(1H,9H)-dione
Click to Show/Hide
|
|||||
| Target(s) | DNA topoisomerase 1 (TOP1) | |||||
| Structure |
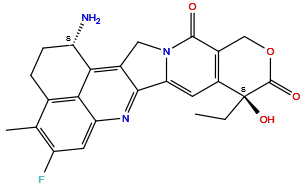
|
|||||
| Formula | C24H22FN3O4 |
|||||
| Isosmiles | [H]O[C@]1(C([H])([H])C([H])([H])[H])C(=O)OC([H])([H])c2c1c([H])c1n(c2=O)C([H])([H])c2c-1nc1c([H])c(F)c(C([H])([H])[H])c3c1c2[C@@]([H])(N([H])[H])C([H])([H])C3([H])[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C24H22FN3O4/c1-3-24(31)14-6-18-21-12(8-28(18)22(29)13(14)9-32-23(24)30)19-16(26)5-4-11-10(2)15(25)7-17(27-21)20(11)19/h6-7,16,31H,3-5,8-9,26H2,1-2H3/t16-,24-/m0/s1
|
|||||
| InChIKey |
ZVYVPGLRVWUPMP-FYSMJZIKSA-N
|
|||||
| IUPAC Name |
(10S,23S)-23-amino-10-ethyl-18-fluoro-10-hydroxy-19-methyl-8-oxa-4,15-diazahexacyclo[14.7.1.02,14.04,13.06,11.020,24]tetracosa-1,6(11),12,14,16,18,20(24)-heptaene-5,9-dione
|
|||||
| Pharmaceutical Properties | Molecule Weight |
435.455 |
Polar area |
107.44 |
||
Complexity |
435.1594344 |
xlogp Value |
2.47312 |
|||
Heavy Count |
32 |
Rot Bonds |
5 |
|||
Hbond acc |
7 |
Hbond Donor |
2 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.16 | nM |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.25 | nM |
CCRF-CEM cells
|
T acute lymphoblastic leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.49 | nM |
DU145 cells
|
Prostate carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.58 | nM |
DMS 114 cells
|
Lung small cell carcinoma
|
[1] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
PRO1184 [Phase 3]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05579366 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1/2 study of PRO1184 in patients with locally advanced and/or metastatic solid tumors.
|
||||
PRO-1160 [Phase 1/2]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05721222 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1/2 study of PRO1160 in patients with renal cell carcinoma (RCC), nasopharyngeal carcinoma (NPC), or non-Hodgkin lymphoma (NHL).
|
||||
BAT8009 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05405621 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1, multi-center, open-label study to assess safety, tolerability, pharmacokinetics, and preliminary efficacy of BAT8009 in patients with advanced solid tumours.
|
||||
BAT8006 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05378737 | Phase Status | Phase 1 | ||
| Clinical Description |
A multicenter, open phase 1 clinical study to evaluate the safety, tolerability, and pharmacokinetic characteristics of BAT8006 for injection in patients with advanced solid tumors.
|
||||
AMT-562 [Phase 1]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.80% (Day 28) | Low HER3 expression (HER3+) | ||
| Method Description |
AMT-562 (10 mg/kg, day 1) induces efficient tumor cell killing in cell line-derived models of Pancreatic cancer cell with HER3 expression with high expression.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-200930) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Low HER3 expression (HER3+) | ||
| Method Description |
AMT-562 (10 m ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of Pancreatic cancer cell with HER3 expression with high expression.
|
||||
| In Vivo Model | Squamous cell carcinoma PDX model (PDX: PDX-361318) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 49) | Low HER3 expression (HER3+) | ||
| Method Description |
AMT-562 (10 m ug/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of Pancreatic cancer cell with HER3 expression with high expression.
|
||||
| In Vivo Model | Pancreatic cancer PDX model (PDX: PDX-361319) | ||||
Trastuzumab-T1000-exatecan [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.00% (Day 27) | Negative HER2 expression (HER2 -) | ||
| Method Description |
T moiety (We conducted a four-week (day 1, 8, 15, 22, and 29) intermittent intravenous dose toxicity study of exatecan mesylate in rats (six animals/group) with a four-week recovery period (three of the six animals).
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
