Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0RJKWE
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
STRO-001
|
|||||
| Synonyms |
BN301; SP-7219; SP-7675; SP-7676; STRO 001; STRO- 001; STRO-001
Click to Show/Hide
|
|||||
| Organization |
Sutro Biopharma, Inc.; BioNova Pharmaceuticals (Shanghai) Ltd.; Piramal Pharma Solutions, Inc.
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
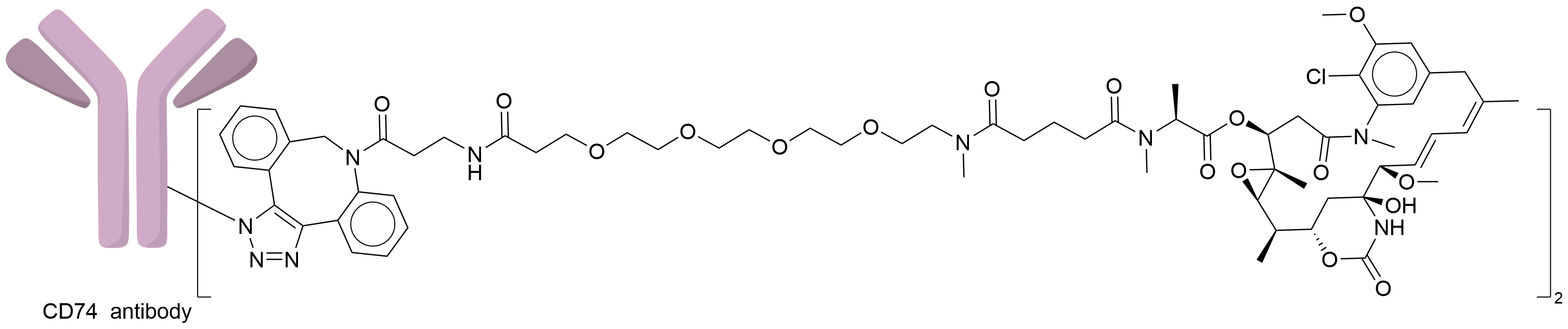
|
|||||
| Antibody Name |
Bezetabart
|
Antibody Info | ||||
| Antigen Name |
HLA class II histocompatibility antigen gamma chain (CD74)
|
Antigen Info | ||||
| Payload Name |
Maytansinoid derivative
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Cys-12 ADC linker
|
Linker Info | ||||
| Conjugate Type |
Enzymatic Catalysis
|
|||||
| Combination Type |
debotansine
|
|||||
| Special Approval(s) |
Orphan drug(FDA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
