Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0RHAZG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Ladiratuzumab vedotin
|
|||||
| Synonyms |
Ladiratuzumab vedotin; MK-6440; SGN-LIV1A; SGN-LIV1A, MK-6440
Click to Show/Hide
|
|||||
| Organization |
Seagen Inc.; F. Hoffmann-La Roche Ltd.; Merck Sharp & Dohme Corp.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 6 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
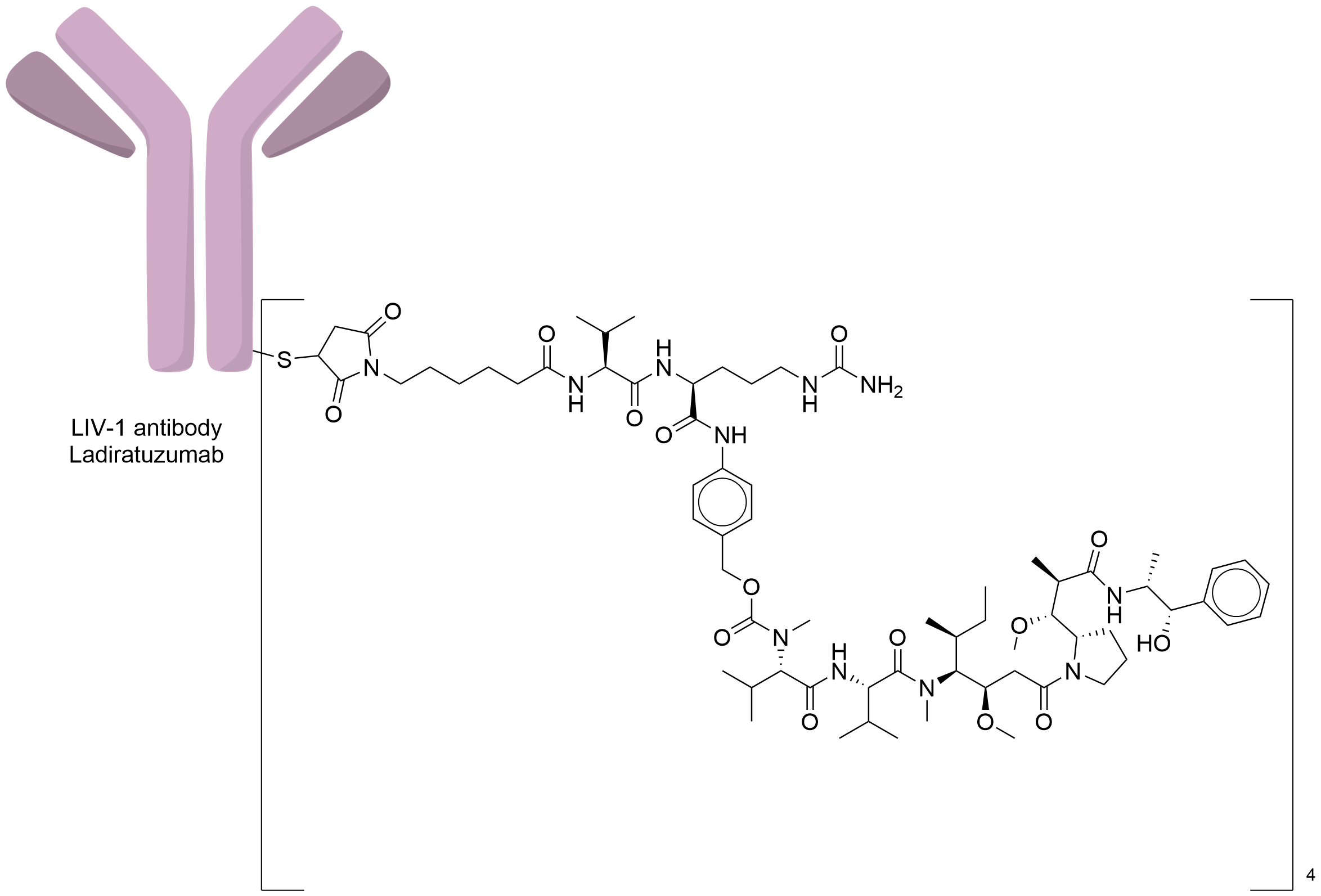
|
|||||
| Antibody Name |
Ladiratuzumab
|
Antibody Info | ||||
| Antigen Name |
Zinc transporter ZIP6 (SLC39A6)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Revealed Based on the Cell Line Data
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Effective Concentration (EC50) |
6.3
|
ng/mL
|
MCF-7 cells
|
Invasive breast carcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
35.00%
|
|||
| Patients Enrolled |
Unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC). Patients must have measureable disease per RECIST v1.1, an ECOG score of 0 or 1, and no prior cytotoxic or anti-PD-L1 treatment for advanced disease.
|
||||
| Administration Dosage |
LV 2.50 mg/kg + pembrolizumab 200 mg intravenously every three weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03310957 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Single arm, open label phase 1b/2 study of SGN-LIV1A in combination with pembrolizumab for first-line treatment of patients with unresectable locally-advanced or metastatic triple-negative breast cancer. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
32.00%
|
|||
| Patients Enrolled |
Women with LIV-1-positive, unresectable, locally advanced or metastatic breast cancer (LA/MBC).
|
||||
| Administration Dosage |
Received a median of 3 cycles (range, 112) of SGN-LIV1A at doses of 0.50-2.80 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01969643 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label, dose-escalation study to evaluate the safety and tolerability of SGN-LIV1A in patients with metastatic breast cancer. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04032704 | Clinical Status | Phase 2 | ||
| Clinical Description | Open-label phase 2 study of ladiratuzumab vedotin (LV) for unresectable locally advanced or metastatic solid tumors. | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
6.30 ng/mL
|
|||
| Method Description |
The inhibitory activity of SGN-LIV1A against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 5 days.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
References
