Linker Information
General Information of This Linker
| Linker ID |
LIN0NBUDP
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
AJICAP
|
|||||
| Linker Type |
Peptide-based site-specific conjugation linker
|
|||||
| Antibody-Linker Relation |
Uncleavable
|
|||||
| Structure |
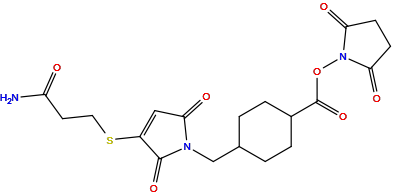
|
|||||
| Formula |
C19H23N3O7S
|
|||||
| Isosmiles |
NC(=O)CCSC1=CC(=O)N(CC2CCC(C(=O)ON3C(=O)CCC3=O)CC2)C1=O
|
|||||
| InChI |
InChI=1S/C19H23N3O7S/c20-14(23)7-8-30-13-9-17(26)21(18(13)27)10-11-1-3-12(4-2-11)19(28)29-22-15(24)5-6-16(22)25/h9,11-12H,1-8,10H2,(H2,20,23)
|
|||||
| InChIKey |
BSYHAJOCHZXFFP-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
437.474
|
Polar area
|
144.15
|
||
|
Complexity
|
30
|
xlogp Value
|
0.2613
|
|||
|
Heavy Count
|
30
|
Rot Bonds
|
8
|
|||
|
Hbond acc
|
8
|
Hbond Donor
|
1
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Trastuzumab-AJICAP-maytansinoid [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Minimal Effective Dose (MED) | < 5.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: three trastuzumab-AJICAP-maytansinoid groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Maximum Tolerated Dose (MTD) | > 120.00 mg/kg | High HER2 expression (HER2+++) | ||
| Method Description |
Following the acclimatization period (1 week), the animals were stratified by body weight and randomly assigned to the following group: three trastuzumab-AJICAP-maytansinoid groups, treated with 20 mg/kg, 60 mg/kg, and 120 mg/kg. Each group consisted of five animals for blood chemistry test as well as five animals for clinical signs and body weight measurements.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
References
