Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0NNKAQ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Ifinatamab deruxtecan
|
|||||
| Synonyms |
DS 7300; DS-7300; DS-7300a; DS7300; Ifinatamab deruxtecan; I-DXd
Click to Show/Hide
|
|||||
| Organization |
Daiichi Sankyo Co., Ltd.
|
|||||
| Drug Status |
Phase 3
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
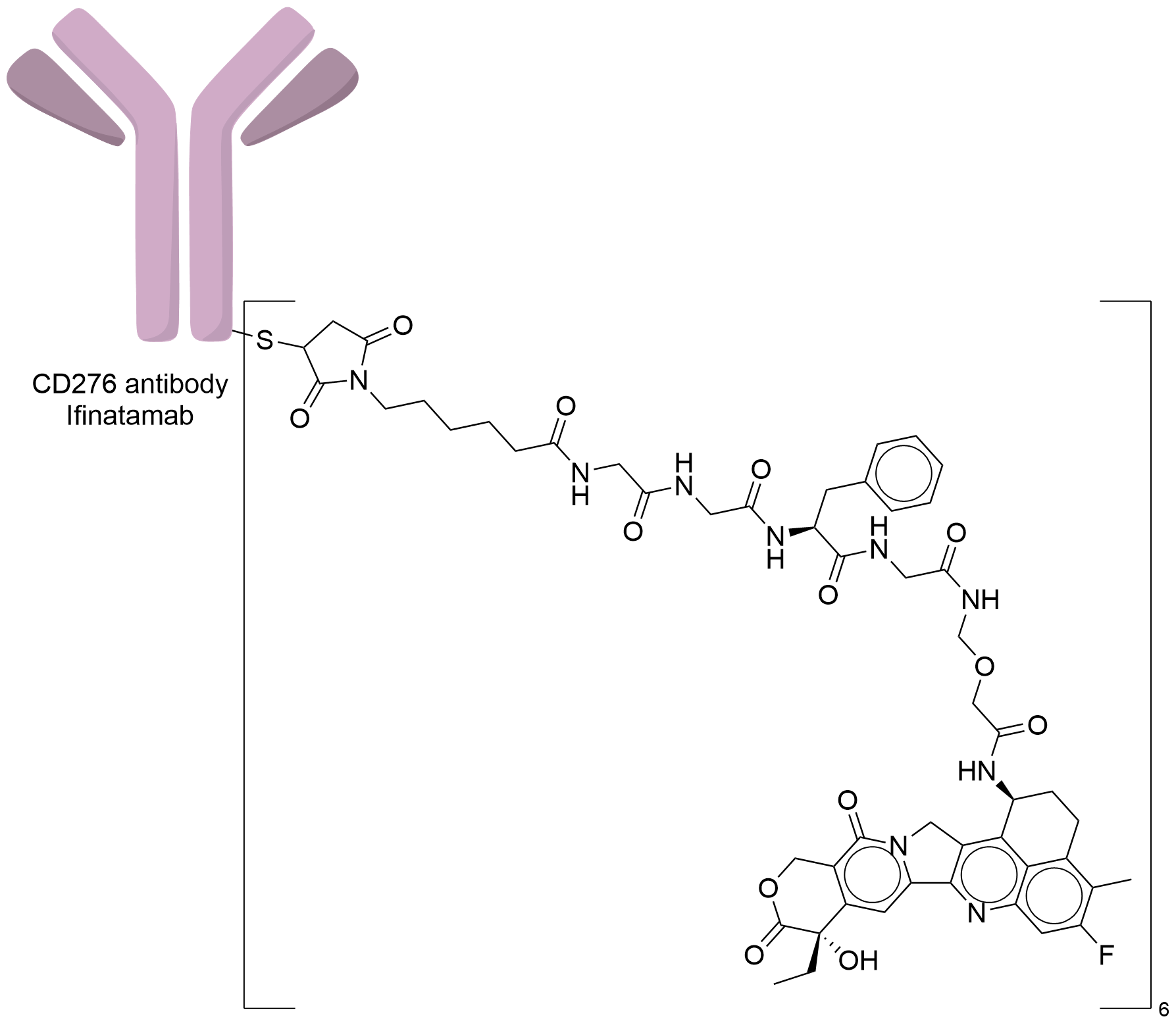
|
|||||
| Antibody Name |
Ifinatamab
|
Antibody Info | ||||
| Antigen Name |
CD276 antigen (CD276)
|
Antigen Info | ||||
| Payload Name |
DX-8951 derivative (DXd)
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
deruxtecan
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
| Standard Type | Value | Units | Animal Model (No. of PDX) |
|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 100
|
%
|
Small cell lung cancer PDX model (PDX: CTG-2093)
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05280470 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2, multicenter, randomized, open-label study of DS-7300a, a B7-H3 antibody drug conjugate (ADC), in subjects with pretreated extensive-stage small cell lung cancer (ES-SCLC). | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04145622 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1/2, two-part, multicenter first-in-human study of DS-7300a in subjects with advanced solid malignant tumors. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 26) | High CD276 expression (CD276+++) | ||
| Method Description |
PDX studies CTG-2093, CTG-0166, CTG-0820, and CTG-1061 studies were performed by Champions Oncology, Inc. Models were established by inoculating tumor fragments derived from patients with small cell lung cancer (SCLC), nonsmall cell lung cancer (NSCLC), head and neck cancer, and bladder cancer, respectively, which were maintained in host mice, subcutaneously into female Hsd: Athymic Nude-Foxn1nu mice.Group assignment was carried out when the tumor volume reached approximately 100 to 300 mm3. The tumor-bearing mice were treated with DS-7300a or relevant controls intravenously on days 0 and 14.
Click to Show/Hide
|
||||
| In Vivo Model | Small cell lung cancer PDX model (PDX: CTG-2093) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.36 nM
|
|||
| Method Description |
In vivo The target specificity and species cross-reactivity of DS-7304a were assessed. Its pharmacologic activities were evaluated in several human cancer cell lines in vitro and xenograft mouse models, including patient-derived xenograft (PDX) mouse models.
|
||||
| In Vitro Model | Endometrial adenocarcinoma | MFE-280 cells | CVCL_1405 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.37 nM
|
|||
| Method Description |
In vivo The target specificity and species cross-reactivity of DS-7303a were assessed. Its pharmacologic activities were evaluated in several human cancer cell lines in vitro and xenograft mouse models, including patient-derived xenograft (PDX) mouse models.
|
||||
| In Vitro Model | Alveolar rhabdomyosarcoma | Rh41 cells | CVCL_2176 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.55 nM
|
|||
| Method Description |
In vivo The target specificity and species cross-reactivity of DS-7302a were assessed. Its pharmacologic activities were evaluated in several human cancer cell lines in vitro and xenograft mouse models, including patient-derived xenograft (PDX) mouse models.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | CCRF-CEM cells | CVCL_0207 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Method Description |
In vivo The target specificity and species cross-reactivity of DS-7300a were assessed. Its pharmacologic activities were evaluated in several human cancer cell lines in vitro and xenograft mouse models, including patient-derived xenograft (PDX) mouse models.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | CCRF-CEM cells | CVCL_0207 | ||
References
