Linker Information
General Information of This Linker
| Linker ID |
LIN0FSRSR
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
AcLys-Val-Cit-PABC
|
|||||
| Linker Type |
Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
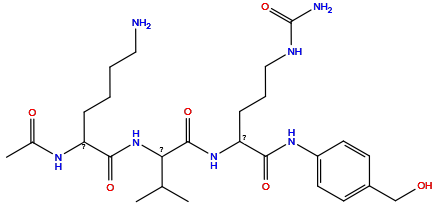
|
|||||
| Formula |
C26H43N7O6
|
|||||
| Isosmiles |
CC(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CCCNC(N)=O)C(=O)Nc1ccc(CO)cc1)C(C)C
|
|||||
| InChI |
InChI=1S/C26H43N7O6/c1-16(2)22(33-24(37)20(30-17(3)35)7-4-5-13-27)25(38)32-21(8-6-14-29-26(28)39)23(36)31-19-11-9-18(15-34)10-12-19/h9-12,16,20-22,34H,4-8,13-15,27H2,1-3H3,(H,30,35)(H,31,36)(H,32,38)(H,33,37)(H3,28,29,39)
|
|||||
| InChIKey |
CCRUWWKFMKVBQV-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
549.673
|
Polar area
|
217.77
|
||
|
Complexity
|
549.3274821
|
xlogp Value
|
-0.1748
|
|||
|
Heavy Count
|
39
|
Rot Bonds
|
17
|
|||
|
Hbond acc
|
7
|
Hbond Donor
|
8
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
PF-06664178 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Advanced solid tumors resistant to standard therapy, or for which no other therapy was available, and at least one measurable lesion defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1).
|
||||
| Administration Dosage |
Once every 21 days as an intravenous infusion over approximately 60 min, doses starting from 0.15 mg/kg to 6.14 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02122146 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, dose escalation study of Pf-06664178 in patients with locally advanced or metastatic solid tumors.
|
||||
| Primary Endpoint |
In the 29 response-evaluable patients,the best overall response observed was limited to stable disease (SD) in 11 patients(37.90%) with PR or CR.
|
||||
| Other Endpoint |
Doses explored ranged from 0.15 mg/kg to 4.80 mg/kg. Doses of 3.60 mg/kg,4.20 mg/kg and 4.80 mg/kg were considered intolerable due to DLTs. MTD and RP2D were not determined.
|
||||
Anti-CXCR4 ADC 381 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 519 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 518 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 554 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.50 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 553 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.10 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 555 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CXCR4 ADC 556 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
39.70 nM
|
High CXCR4 expression (CXCR4+++) | ||
| Method Description |
Cells were seeded in a culture-treated 96-well clear plate and incubated at 37°C under 5% CO2 for 24h. Serially diluted samples (50L) were added to each well and the plate was incubated at 37°C for 72h.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
References
