Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0JBKMD
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Labetuzumab govitecan
|
|||||
| Synonyms |
Anti-CEA monoclonal antibody; Anti-carcinoembryonic antigen monoclonal antibody; HMN-14-SN38; IMMU-130; IMMU-130, hMN14-SN38; Labetuzumab govitecan; Labetuzumab-SN38 conjugate; SN38-labetuzumab conjugate; anti-CEACAM5-SN38; hMN-14-SN38 antibody-drug conjugate
Click to Show/Hide
|
|||||
| Organization |
Immunomedics, Inc.; IBC Pharmaceuticals, Inc.; Gilead Sciences, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
7.6
|
|||||
| Structure |
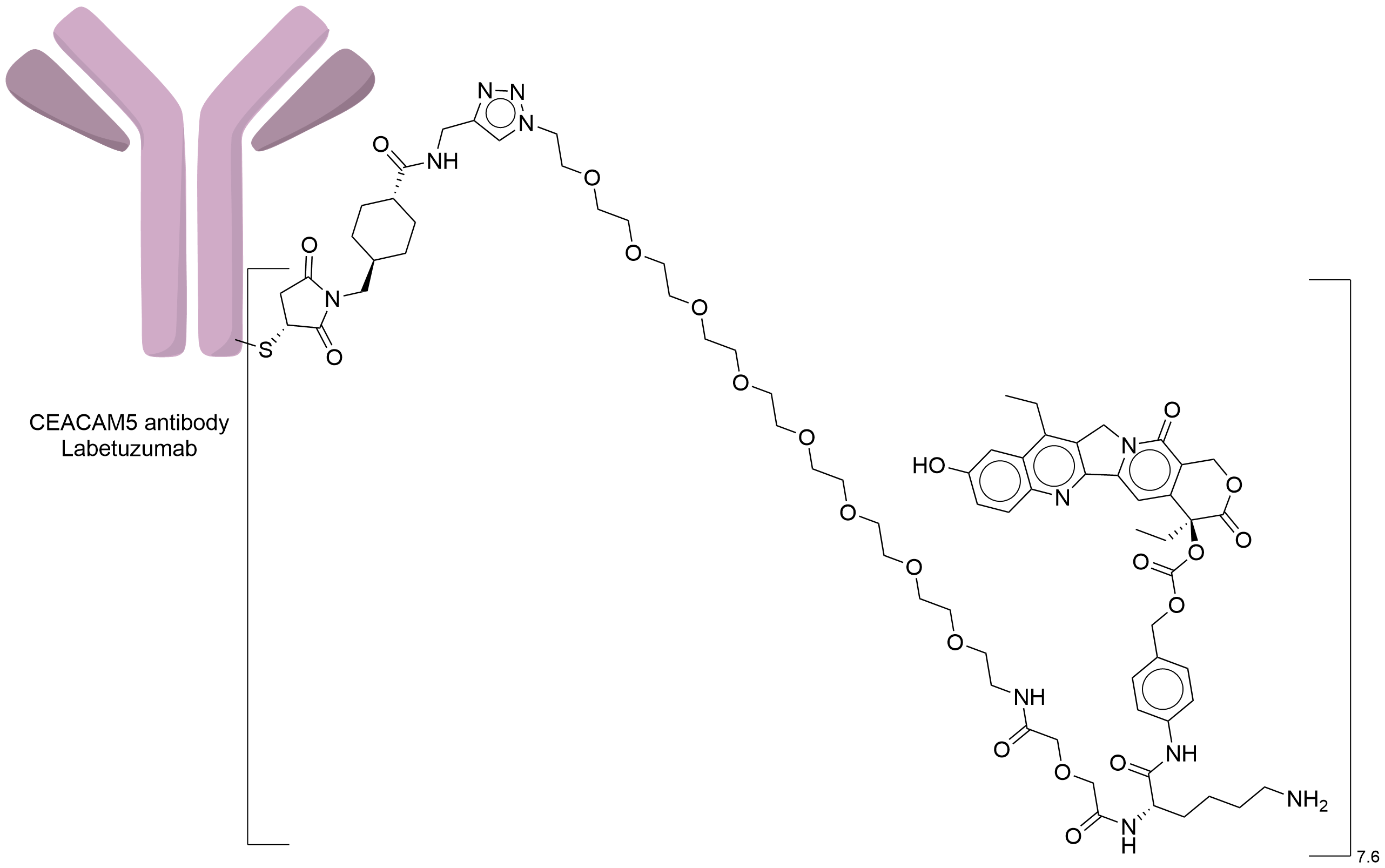
|
|||||
| Antibody Name |
Labetuzumab
|
Antibody Info | ||||
| Antigen Name |
Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5)
|
Antigen Info | ||||
| Payload Name |
Active metabolite of irinotecan SN38
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
CL2A
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
govitecan
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Patients Enrolled |
Relapsed or refractory metastatic colorectal cancer (mCRC) who had received at least one prior irinotecan-containing regimen.
|
||||
| Administration Dosage |
Once weekly at 8 and 10 mg/kg, or two times per week at 4 and 6 mg/km on weeks 1 and 2 of 3-week repeated cycles, intravenous.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01605318 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1/2 study of once or twice weekly IMMU-130 (hMN-14-SN38, antibody-drug conjugate) in patients with colorectal cancer. | ||||
| Primary Endpoint |
The median PFS for all 86 patients was 3.60 months (95% CI,2.00 months to 4.00 months), with 16.8% (14 of 86) remaining progression free for at least 6 months, including three patients who maintained this status for at least 1 year. The median OS was 6.90 months (95% CI, 5.70 months to 7.80 months), with 24.41% (21 of 86) surviving for at least 1 year, including three patients who survived at least 2 years (one for 3 years).
Click to Show/Hide
|
||||
| Other Endpoint |
In the regorafenib subset (n = 23), the median PFS and OS were 3.90 and 6.70 months, respectively.
|
||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.23 nM
|
|||
| Method Description |
The inhibitory activity of IMMU-130 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 4 days.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.04 nM
|
|||
| Method Description |
The inhibitory activity of IMMU-130 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 4 days.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
140.00 nM
|
|||
| Method Description |
The inhibitory activity of IMMU-130 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 4 days.
|
||||
| In Vitro Model | Prostate cancer | MSKCC EF1 cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.32 uM
|
|||
| Method Description |
The inhibitory activity of IMMU-130 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 4 days.
|
||||
| In Vitro Model | Prostate small cell carcinoma | NCI-H660 cells | CVCL_1576 | ||
References
