Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0GVDPA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
40H3-Deruxtecan
|
|||||
| Synonyms |
40H3 Deruxtecan
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
7
|
|||||
| Structure |
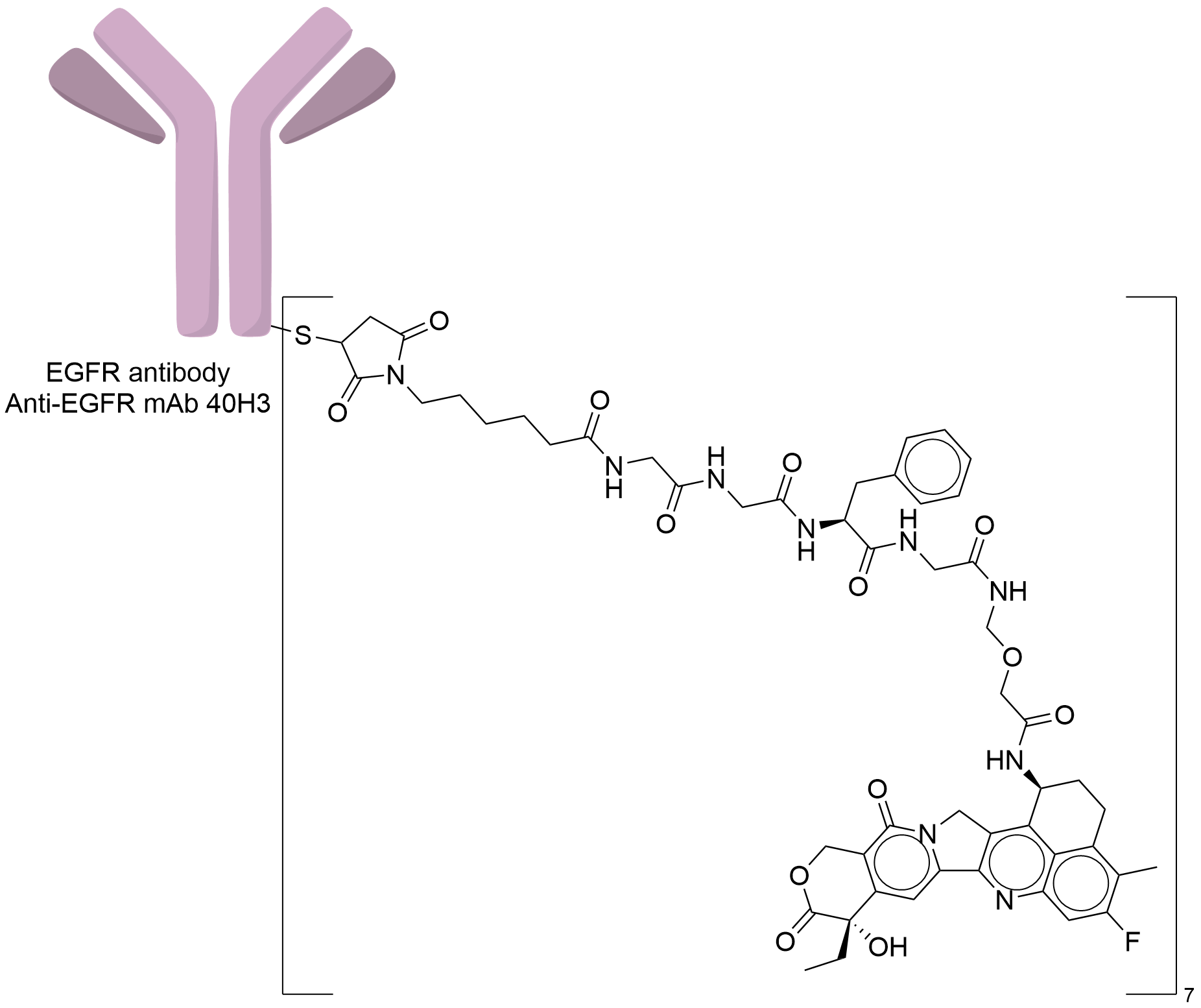
|
|||||
| Antibody Name |
Anti-EGFR mAb 40H3
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
DX-8951 derivative (DXd)
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
The free sulfhydryl group obtained by the reduction of interchain cysteine is site-specific conjugated with maleimide, michael addition reaction.
|
|||||
| Combination Type |
Deruxtecan
|
|||||
General Information of The Activity Data Related to This ADC
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 9.32 nM | High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 70.36 nM | Moderate EGFR expression (EGFR++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | BT-20 cells | CVCL_0178 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 90.21 nM | High EGFR expression (EGFR+++) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Negative EGFR expression (EGFR-) | ||
| Method Description |
1 x104 cells per well in a volume of 100 ul were plated in 96-well tissue culture plates. After 24 h, ADCs were added at the indicated concentrations. After 72 h, the medium was removed and the viability was determined using the CellTiter-Glo luminescent cell viability assay kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
References
