Linker Information
General Information of This Linker
| Linker ID |
LIN0MESCO
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Sulfo-SPDB
|
|||||
| Linker Type |
Flexible dual-reactive (amino/thiol) linker; Thiol-sensitive linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
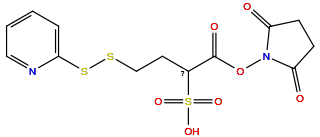
|
|||||
| Formula |
C13H14N2O7S3
|
|||||
| Isosmiles |
[H]OS(=O)(=O)C([H])(C(=O)ON1C(=O)C([H])([H])C([H])([H])C1=O)C([H])([H])C([H])([H])SSc1nc([H])c([H])c([H])c1[H]
|
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C13H14N2O7S3/c16-11-4-5-12(17)15(11)22-13(18)9(25(19,20)21)6-8-23-24-10-3-1-2-7-14-10/h1-3,7,9H,4-6,8H2,(H,19,20,21)
|
|||||
| InChIKey |
FUHCFUVCWLZEDQ-UHFFFAOYSA-N
|
|||||
| IUPAC Name |
1-(2,5-dioxopyrrolidin-1-yl)oxy-1-oxo-4-(pyridin-2-yldisulfanyl)butane-2-sulfonic acid
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
406.463
|
Polar area
|
130.94
|
||
|
Complexity
|
405.9963138
|
xlogp Value
|
1.0756
|
|||
|
Heavy Count
|
25
|
Rot Bonds
|
9
|
|||
|
Hbond acc
|
10
|
Hbond Donor
|
1
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Mirvetuximab soravtansine [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
22.00%
|
High FOLR1 expression (FOLR1+++) | ||
| Patients Enrolled |
Platinum-resistant epithelial ovarian cancer (EOC); Eligible patients with 1-3 prior lines of therapy and whose tumors were positive for FR expression.
|
||||
| Administration Dosage |
6 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02631876 | Clinical Status | Phase 3 | ||
| Clinical Description |
FORWARD I: A randomized, open label phase 3 study to evaluate the safety and efficacy of mirvetuximab soravtansine (IMGN853) versus investigator's choice of chemotherapy in women with folate receptor alpha positive advanced epithelial ovarian cancer, primary peritoneal cancer or fallopian tube cancer.
|
||||
| Primary Endpoint |
For the ITT population, Kaplan-Meier estimates showed no significant difference (HR, 0.98; 95% Cl, 0.73-1.31; P=0.897) in progression-free survival (PFS) between groups Median PFS was 4.10 and 4.40 months for MIRV and IC chemotherapy, respectively. In the prespecified high FR subgroup, PFS was longer in patients in the MIRV group compared with IC chemotherapy (median, 4.80 months versus 3.30 months; HR, 0.69, 95% CI, 0.48 to 1.00; P = 0.049). Based on the Hochberg procedure used in the statistical analysis plan for the study, this P value did not meet statistical significanceBoth in the ITT group and high FR subgroup, OS were not significantly different.
Click to Show/Hide
|
||||
| Other Endpoint |
Mirvetuximab soravtansine vs IC chemotherapy in the ITT group: ORR=22.00% vs 12.00%, P=0.015; cancer antigen-125 (CA-125) responses=51.00% vs 27.00%, P<0001; median PFS2 duration (time from randomization to second disease progression or death)=10.00 vs 8.40 months, HR=0.64, 95% Cl, 0.49-0.84, P<0.001); PRO in the EORTC QLQ-OV28 Abdominal/GI Symptom Subscale=32.00% vs 14.00%, P=0.016; Mirvetuximab soravtansine vs IC chemotherapy in the high FR subset, ORR=24.00% vs 10.00%, P=0.014; cancer antigen-125 (CA-125) responses=53.00% vs 25.00%, P=0.001; median PFS2 duration=10.10 vs 8.40 months, HR=0.56, 95% Cl, 0.39-0.79, P<0.001); PRO in the EORTC QLQ-OV28 Abdominal/GI Symptom Subscale=27.00% vs 13.00%, P=0.143.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
31.73%
|
High FOLR1 expression (FOLR1+++) | ||
| Patients Enrolled |
Platinum-Resistant, Advanced High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancers With High Folate Receptor-Alpha Expression.
|
||||
| Administration Dosage |
6 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04296890 | Clinical Status | Phase 3 | ||
| Clinical Description |
SORAYA: A Phase 3, single arm study of mirvetuximab soravtansine in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression.
|
||||
| Primary Endpoint |
Confirmed Overall Response Rate=31.73% [(N=104, 95% Cl, 22.90-41.60), Complete response rate=4.80%, Partial response rate=26.90%].
|
||||
| Other Endpoint |
Median duration of response=6.90 months (N=33, 95% Cl 5.60-9.70).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
39.00%
|
Moderate FOLR1 expression (FOLR1++) | ||
| Patients Enrolled |
Platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.
|
||||
| Administration Dosage |
6 mg/kg (adjusted ideal body weight) once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02606305 | Clinical Status | Phase 1b/2 | ||
| Clinical Description |
A phase 1b/2 study to evaluate the safety, tolerability and pharmacokinetics of mirvetuximab soravtansine (IMGN853) in combination with bevacizumab, carboplatin, pegylated liposomal doxorubicin, pembrolizumab, or bevacizumab + carboplatin, in adults with folate receptor alpha positive advanced epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer.
Click to Show/Hide
|
||||
| Primary Endpoint |
For AURELIA-type patients (N=16, bevacizumab-naive and 1-2 prior lines of therapy, plus medium/high FRa expression), confirmed ORR=56.00%, median duration of response (mDOR)=12.0 months, median progression-free survival (mPFS) = 9.9 months.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.40% (Day 30) | Moderate ROR1 expression (ROR1++) | ||
| Method Description |
In vivoexperiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts as well as a uterine serous tumor PDX model (BIO (K)1).all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg.
|
||||
| In Vivo Model | Uterine serous carcinomas PDX model (PDX: BIO(K)1) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 25) | Moderate ROR1 expression (ROR1++) | ||
| Method Description |
In vivoexperiments comparing the antitumor activity of IMGN853 versus ADC isotype control, PBS and M9346A were conducted using both high grade endometrioid/clear cell xenografts as well as a uterine serous tumor PDX model (BIO (K)1).all treatments were given twice, one week apart by retro-orbital injection at a concentration of 5 mg/kg.
|
||||
| In Vivo Model | Uterine serous carcinomas PDX model (PDX: END(K)265) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 33.00% (Day 5) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 45.00% (Day 6) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 64.00% (Day 26) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.00% (Day 40) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OV-90 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OV-90 CDX model | ||||
| In Vitro Model | Ovarian adenocarcinoma | OV-90 cells | CVCL_3768 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 82.00% (Day 21) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 25 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
93.00% (Day 32)
|
High FOLR1 expression (FOLR1+++; 1,300,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 7 after cell inoculation.
|
||||
| In Vivo Model | Ovarian carcinoma Igrov-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.00% (Day 41) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.00% (Day 67) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of IGROV-1 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | IGROV-1 CDX model | ||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
99.07% (Day 30)
|
High FOLR1 expression (FOLR1+++; 4,500,000 FOLR1 molecules/cell) | ||
| Method Description |
Animals with established tumors of about 130 mm3 were treated with intravenous single injection of the M9346A-DM conjugates at 2.5±0.2 mg/kg, equivalent to 51±3 ug conjugated maytansinoid per kg The conjugates were injected on day 20 after cell inoculation.
|
||||
| In Vivo Model | FRalpha-positive KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 75) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 50 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 120) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of OVCAR-3 cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 100 ug/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15±0.08 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20±0.10 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00±0.40 nM
|
|||
| Method Description |
Dilutions of conjugates or unconjugated maytansinoid in the appropriate culture medium were added to wells of 96-well flat-bottomed plates containing 1 x103 cells per well The plates were incubated at 37°C, 6% CO2 for either 5 days (continuos exposure) or for 4 hours followed by 5-day incubation in conjugate-free medium (short exposure). Cell viability was determined by the WST-8 assay in accordance with the manufacturer's protocol, and IC50 were generated using a sigmoidal dose-response (variable slope) nonlinear regression curve fit.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.20 ug/mL
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FOLR1 expression.
|
||||
| In Vitro Model | Endometrial undifferentiated carcinoma | END-2 cells | CVCL_B5KE | ||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
74.60 ng/mL
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell cytotoxicity was tested with IMGN853, ADC isotype control and M9346A in pure and mixed endometrial endometrioid cell lines harboring high FOLR1 expression.
|
||||
| In Vitro Model | Endometrial undifferentiated carcinoma | END-1 cells | CVCL_B5JE | ||
HKT-288 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Patients Enrolled |
Advanced (metastatic or locally advanced) serous epithelial ovarian, serous fallopian tubal or serous primary peritoneal cancer or advanced clear cell or papillary renal cell carcinoma (RCC), who had received or were intolerant to all therapies known to confer clinical benefit for their disease and Eastern Cooperative Oncology Group (ECOG) performance status 2.
Click to Show/Hide
|
||||
| Administration Dosage |
HKT288 was administered intravenously (IV) every 3 weeks until patients experienced unacceptable toxicity or progressive disease (PD). The starting dose of 0.30 mg/kg was determined based on the highest nonseverely toxic dose in monkeys, which was 2 mg/kg IV weekly.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02947152 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, multicenter, open-label dose escalation and expansion study of HKT288, administered intravenously in adult patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
| Primary Endpoint |
The best overall response on the 0.30 mg/kg cohort in patients with measurable disease was RECIST v1.1 stable disease in 3 patients and PD in 2 patients.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [8] | ||||
| Patients Enrolled |
Patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
| Administration Dosage |
Cadherin-6-targeting ADC iv at dose of 0.30, 0.75 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02947152 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, multicenter, open-label dose escalation and expansion study of HKT288, administered intravenously in adult patients with advanced solid tumors, including epithelial ovarian cancer and renal cell carcinoma.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-sulfo-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 45) | High CDH6 expression (CDH6+++) | ||
| Method Description |
CDH6-sulfo-DM4 induces efficient tumor cell killing in cell PDX models with CDH6 expression.
|
||||
| In Vivo Model | Ovarian cancer CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
IMGN-779 [Phase 1 (Terminated)]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 15) | Positive CD33 expression (CD33+++/++) | ||
| Method Description |
EOL-1 tumor-bearing mice received a single intravenous bolus administration of vehicle, IMGN779, or Ab-DGN462, with each conjugate molecule dosed at approximately 1.5 mg/kg ADC by antibody concentration (i.e., 10 or 30 ug/kg linked IGN).
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 30) | Positive CD33 expression (CD33+++/++) | ||
| Method Description |
IMGN779 (0.5 mg/kg, a single dose) induces efficient tumor cell killing in cell line-derived models of MV4-11 cells with CD33 expression with high expression.
|
||||
| In Vivo Model | MV4-11 CDX model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | MV4-11 cells | CVCL_0064 | ||
NOV0712-DM4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 13) | Negative CDH6 expression (CDH6-) | ||
| Method Description |
The CDH6-negative human ovarian tumor cell line ES-2 was subcutaneously inoculated at a dose of 1,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 7 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | ES-2 CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | ES-2 cells | CVCL_3509 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 13) | Negative CDH6 expression (CDH6-) | ||
| Method Description |
The CDH6-negative human ovarian tumor cell line ES-2 was subcutaneously inoculated at a dose of 1,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 7 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | ES-2 CDX model | ||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | ES-2 cells | CVCL_3509 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 3.10% (Day 28) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line OVCAR-3 was subcutane-ously inoculated at a dose of 10,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 22 of grouping, theantibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 6.77% (Day 21) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line PA-1 was subcutaneously inoculatedat a dose of 8,500,000 cells to the right flank region of eachfemale nude mouse (Day 0). On the day 11 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 9.52% (Day 23) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human renal cell tumor cell line786-0 was subcutaneously inoculated at a dose of 5,000,000 cells to the right flank regionof each male SCID mouse (Day 0). On the day 20 of grouping, the anti-body-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | 786-O CDX model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 12.03% (Day 21) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line PA-1 was subcutaneously inoculatedat a dose of 8,500,000 cells to the right flank region of eachfemale nude mouse (Day 0). On the day 11 of grouping, the antibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 1 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 23.91% (Day 23) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human renal cell tumor cell line786-0 was subcutaneously inoculated at a dose of 5,000,000 cells to the right flank regionof each male SCID mouse (Day 0). On the day 20 of grouping, the anti-body-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to thetail of each mouse.
|
||||
| In Vivo Model | 786-O CDX model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.02% (Day 24) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human renal cell tumor cell line 786-O was subcutaneously inoculated at a dose of 5,000,000 cells to the right flank regionof each male SCID mouse (Day 0). On the day 18 of grouping, NOV0712-DM4 was intravenously administered at a dose of 3 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | 786-O CDX model | ||||
| In Vitro Model | Renal cell carcinoma | 786-O cells | CVCL_1051 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.30% (Day 28) | Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
The CDH6-positive human ovarian tumor cell line OVCAR-3 was subcutane-ously inoculated at a dose of 10,000,000 cells to the right flank region of each female nude mouse (Day 0). On the day 22 of grouping, theantibody-drug conjugate NOV0712-DM4 was intravenously administered at doses of 3 mg/kg to the tail of each mouse.
|
||||
| In Vivo Model | OVCAR-3 CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.00-10.00 nM
|
Positive CDH6 expression (CDH6+++/++) | ||
| Method Description |
CDH6-positive human ovarian tumor cell line PA-1 was seeded over a 96-well plate at 2,000 cells/100 L/well in MEM medium supplemented with 10% FBS, and the cells were then cultured overnight. On the next day, each of the 4 humanized hG019-drug conjugates or NOV0712-DM4 was added to the cells.
|
||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
huMov19-sulfo-SPDB-DM4 (DAR6.8) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.72% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
huMov19-sulfo-SPDB-DM4 (DAR3.8) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.08% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 2.5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 17) | Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugates of the exemplary anti-FOLR1 antibodies were tested using an established xenograft model of KB cells implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once on day 6 post cell inoculation with 5 mg/kg of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | KB CDX model | ||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Human papillomavirus-related endocervical adenocarcinoma | KB cells | CVCL_0372 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive FOLR1 expression (FOLR1 +++/++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.00 nM
|
Moderate FOLR1 expression (FOLR1 ++) | ||
| Method Description |
Conjugate in various concentrations was added to FOLR1-expressing cells in a 96 well plate at 1,000 cells per well in 100 pL in complete RPMI medium.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
Farletuzumab-sulfo SPDB-DM4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
High FOLR1 expression(FOLR1+++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Ovarian endometrioid adenocarcinoma | IGROV-1 cells | CVCL_1304 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.49 nM
|
Moderate FOLR1 expression(FOLR1++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.52 nM
|
Moderate FOLR1 expression(FOLR1++) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Lung non-small cell carcinoma | NCI-H2110 cells | CVCL_1530 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.85 nM
|
Low FOLR1 expression(FOLR1+) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.28 nM
|
Negative FOLR1 expression(FOLR1-) | ||
| Method Description |
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
References
