Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0GGKEV
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
NBE-002
|
|||||
| Synonyms |
BI-3702025; NBE 002; NBE-002; NBE-002 ; PNU-159682-anti-ROR1 antibody drug conjugate; huXBR1-402-PNU
Click to Show/Hide
|
|||||
| Organization |
NBE-Therapeutics AG
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.7-3.9
|
|||||
| Structure |
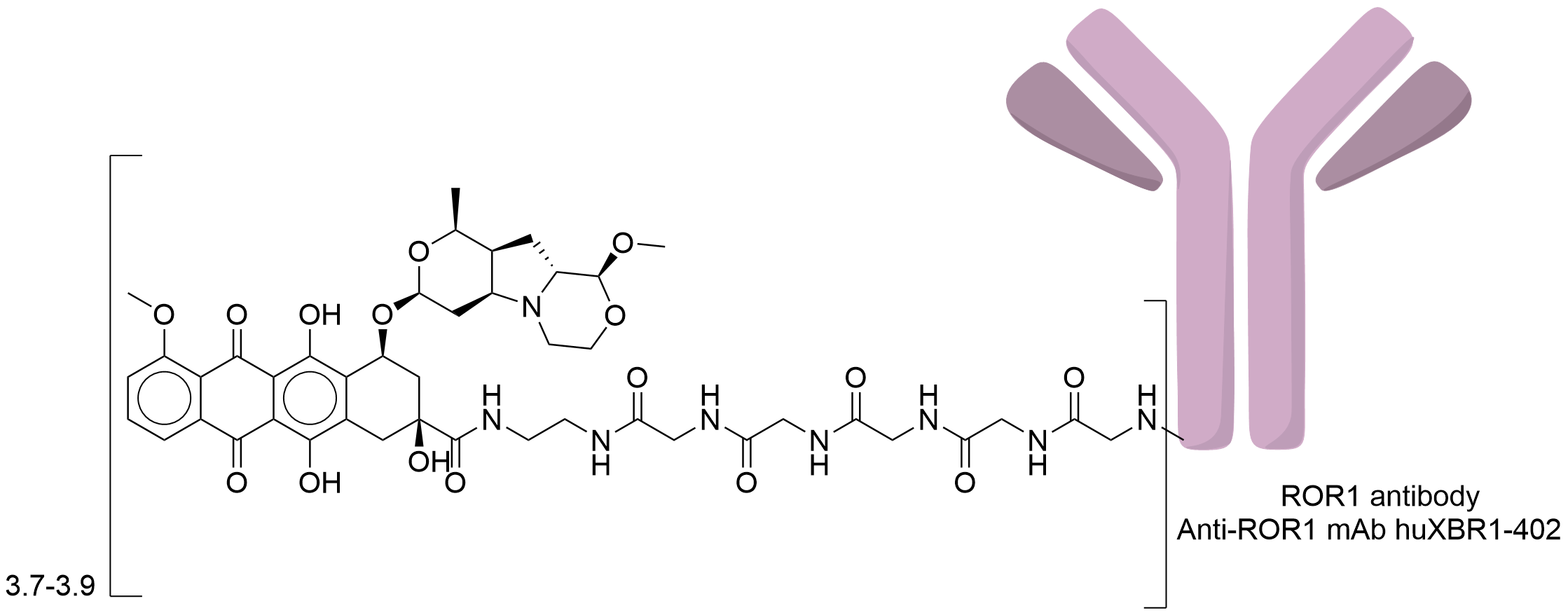
|
|||||
| Antibody Name |
Anti-ROR1 mAb huXBR1-402
|
Antibody Info | ||||
| Antigen Name |
Inactive tyrosine-protein kinase transmembrane receptor ROR1 (ROR1)
|
Antigen Info | ||||
| Payload Name |
PNU-159682
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 2-alpha (TOP2A)
|
Target Info | ||||
| Linker Name |
LPETG-Gly5-EDA
|
Linker Info | ||||
| Conjugate Type |
Enzymatic Catalysis
|
|||||
| DrugMap ID | ||||||
| TTD ID | ||||||
