Payload Information
General Information of This Payload
| Payload ID | PAY0WKBCR |
|||||
|---|---|---|---|---|---|---|
| Name | PNU-159682 |
|||||
| Synonyms |
PNU-159682; 202350-68-3; UNII-CQ5A9ZNT7C; CQ5A9ZNT7C; PNU159682; PNU 159682; (8S,10S)-6,8,11-Trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-10-(((1S,3R,4aS,9S,9aR,10aS)-9-methoxy-1-methyloctahydro-1H-pyrano[4',3':4,5]oxazolo[2,3-c][1,4]oxazin-3-yl)oxy)-7,8,9,10-tetrahydrotetracene-5,12-dione; (7S,9S)-6,9,11-Trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-7-[[(2S,4R,6S,7S,9R,10S)-10-methoxy-6-methyl-5,8,11-trioxa-1-azatricyclo[7.4.0.02,7]tridecan-4-yl]oxy]-8,10-dihydro-7H-tetracene-5,12-dione; (8S,10S)-7,8,9,10-Tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-10-(((1S,3R,4aS,9S,9aR,10aS)-octahydro-9-methoxy-1-methyl-1H-pyrano(4',3':4,5)oxazolo(2,3-C)(1,4)oxazin-3-yl)oxy)-5,12-naphthacenedione; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-10-(((1S,3R,4aS,9S,9aR,10aS)-octahydro-9-methoxy-1-methyl-1H-pyrano(4',3':4,5)oxazolo(2,3-C)(1,4)oxazin-3-yl)oxy)-, (8S,10S)-; (8S,10S)-7,8,9,10-Tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-10-[[(1S,3R,4aS,9S,9aR,10aS)-octahydro-9-methoxy-1-methyl-1H-pyrano[4',3':4,5]oxazolo[2,3-c][1,4]oxazin-3-yl]oxy]-5,12-naphthacenedione; SCHEMBL3801318; CHEMBL4777727; SLURUCSFDHKXFR-WWMWMSKMSA-N; EX-A3364; CS-3298; BP-29359; HY-16700; MS-30920; A903771; (8S,10S)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-10-{[(2S,4R,6S,7S,9R,10S)-10-methoxy-6-methyl-5,8,11-trioxa-1-azatricyclo[7.4.0.0,tridecan-4-yl]oxy}-5,7,8,9,10,12-hexahydrotetracene-5,12-dione; (8S,10S)-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-10-{[(1S,3R,4aS,9S,9aR,10aS)-9-methoxy-1-methyloctahydro-1H-pyrano[4',3':4,5] [1,3] oxazolo[2,3-c][1,4]oxazin-3-yl]oxy}-7,8,9,10-tetrahydrotetracene-5,12-dione; (8S,10S)-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-10-{[(1S,3R,4aS,9S,9aR,10aS)-9-methoxy-1-methyloctahydro-1H-pyrano[4',3':4,5][1,3]oxazolo[2,3-c][1,4]oxazin-3-yl]oxy}-7,8,9,10-tetrahydrotetracene-5,12-dione
Click to Show/Hide
|
|||||
| Target(s) | DNA topoisomerase 2-alpha (TOP2A) | |||||
| Structure |
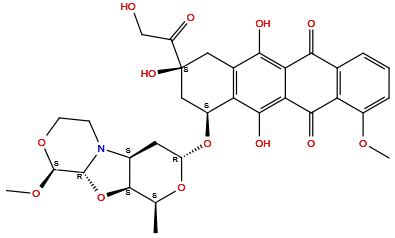
|
|||||
| Formula | C32H35NO13 |
|||||
| Isosmiles | [H]Oc1c2c(c(O[H])c3c1C([H])([H])[C@@](O[H])(C(=O)C([H])([H])O[H])C([H])([H])[C@]3([H])O[C@]1([H])O[C@@]([H])(C([H])([H])[H])[C@@]3([H])O[C@@]4([H])N(C([H])([H])C([H])([H])O[C@]4([H])OC([H])([H])[H])[C@@]3([H])C1([H])[H])C(=O)c1c(OC([H])([H])[H])c([H])c([H])c([H])c1C2=O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C32H35NO13/c1-13-29-16(33-7-8-43-31(42-3)30(33)46-29)9-20(44-13)45-18-11-32(40,19(35)12-34)10-15-22(18)28(39)24-23(26(15)37)25(36)14-5-4-6-17(41-2)21(14)27(24)38/h4-6,13,16,18,20,29-31,34,37,39-40H,7-12H2,1-3H3/t13-,16-,18-,20-,29+,30+,31-,32-/m0/s1
|
|||||
| InChIKey |
SLURUCSFDHKXFR-WWMWMSKMSA-N
|
|||||
| IUPAC Name |
(7S,9S)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-7-[[(2S,4R,6S,7S,9R,10S)-10-methoxy-6-methyl-5,8,11-trioxa-1-azatricyclo[7.4.0.02,7]tridecan-4-yl]oxy]-8,10-dihydro-7H-tetracene-5,12-dione
|
|||||
| Pharmaceutical Properties | Molecule Weight |
641.626 |
Polar area |
190.75 |
||
Complexity |
641.2108402 |
xlogp Value |
0.7113 |
|||
Heavy Count |
46 |
Rot Bonds |
13 |
|||
Hbond acc |
14 |
Hbond Donor |
4 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
SOT-102 [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05525286 | Phase Status | Phase 1 | ||
| Clinical Description |
A multicentric phase 1/2 trial to evaluate the safety and efficacy of SOT102 as monotherapy and in combination with standard of care treatment in patients with gastric and pancreatic adenocarcinoma.
|
||||
HuIgG1-19 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 13.33% (Day 30 | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
In vivo efficacy of PNU-conjugated ADCs in NSCLC LU253 PDX subcutaneous models in NOD/SCID mice. A single dose of 1.0 mg/kg HuIgG1-19 ADC.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: LU253 PDX) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 21.34% (Day 15) | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
In vivo efficacy of PNU-conjugated ADCs in colorectal CR188 PDX subcutaneous models in NOD/SCID mice. A single dose of 1.0 mg/kg HuIgG1-19 ADC.
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR188 PDX) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 3.00 nM | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
3.80 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
hCD46-19 [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 52.34% (Day 30) | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
In vivo efficacy of PNU-conjugated ADCs in NSCLC LU253 PDX subcutaneous models in NOD/SCID mice. A single dose of 0.5 mg/kg hCD46-19 ADC.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: LU253 PDX) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.34% (Day 30) | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
In vivo efficacy of PNU-conjugated ADCs in colorectal CR188 PDX subcutaneous models in NOD/SCID mice. A single dose of 0.5 mg/kg hCD46-19 ADC.
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR188 PDX) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.67% (Day 30) | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
In vivo efficacy of PNU-conjugated ADCs in colorectal CR188 PDX subcutaneous models in NOD/SCID mice. A single dose of 1.0 mg/kg hCD46-19 ADC.
|
||||
| In Vivo Model | Colorectal cancer PDX model (PDX: CR188 PDX) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.48% (Day 30) | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
In vivo efficacy of PNU-conjugated ADCs in NSCLC LU253 PDX subcutaneous models in NOD/SCID mice. A single dose of 1.0 mg/kg hCD46-19 ADC.
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: LU253 PDX) | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
47.00 pM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 3.00 nM | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
Trastuzumab-PNUEDAGly5 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
1,000,000 EMT6 mouse breast cancer cells expressing human HER-2 previously determined to be suitable for in vivo growth, were implanted into theright mammary fat pads of female Balb/c mice. Animals were treated onthe same day (day 13) and 7 days later (day 20) byintravenous injection of the reference ADC Kadcyla (15 mg/kg), Trastuzumab-PNU-EDA-Glys (1 mg/kg) or vehicle control.
Click to Show/Hide
|
||||
| In Vivo Model | EMT6 CDX model (Expressing hHER2) | ||||
| In Vitro Model | Mammary gland malignant neoplasms | EMT6 cells (High HER2 expression) | CVCL_1923 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
6.90 ng/mL
|
High CD30 expression (CD30 +++) | ||
| Method Description |
Dose response of the cytotoxic effects of the indicated ADCs on human Non-Hodgkin lymphoma cell line Karpas-299, and on human Hodgkin lymphoma cell line L428 cells.
|
||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | < 10.00 ug/mL | Low CD30 expression (CD30 +) | ||
| Method Description |
Dose response of the cytotoxic effects of the indicated ADCs on human Non-Hodgkin lymphoma cell line Karpas-299, and on human Hodgkin lymphoma cell line L428 cells.
|
||||
| In Vitro Model | Hodgkin lymphoma | L-428 cells | CVCL_1361 | ||
Tras-Gly5-EDA-Pnu [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 26) | High HER2 expression (HER2+++; 170,000 HER2 molecules/cell) | ||
| Method Description |
JIMT-1 cells were transplanted subcutaneously into CB17.SCID mice and tumors were allowed to grow to a volume of 100-150 mm3. Mice were then treated intravenously 3 times weekly with the 1 mg/kg ADC preparations.
|
||||
| In Vivo Model | Trastuzumab-resistant breast cancer CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.70 ng/mL
|
High HER2 expression (HER2+++; 694,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
14.70 ng/mL
|
Moderate HER2 expression (HER2++; 32,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
CAC10-Gly5-PNU [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 16) | High CD30 expression (CD30+++) | ||
| Method Description |
CD30 Karpas-299 cells were either transplanted subcutaneously into NSG, or into CB17.SCID mice. Mice were treated intravenously 3 times weekly with 1 mg/kg preparations, beginning one day post randomization, when the tumors had reached a size ranging between 100 and 150 mm3.
|
||||
| In Vivo Model | Non-Hodgkin's lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 14) | High CD30 expression (CD30+++) | ||
| Method Description |
CD30 Karpas-299 cells were either transplanted subcutaneously into NSG, or into CB17.SCID mice. Mice were treated intravenously 3 times weekly with 1 mg/kg preparations, beginning one day post randomization, when the tumors had reached a size ranging between 100 and 150 mm3.
|
||||
| In Vivo Model | Non-Hodgkin's lymphoma CDX model | ||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.80 ng/mL
|
High CD30 expression (CD30+++) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | ALK-positive anaplastic large cell lymphoma | Karpas-299 cells | CVCL_1324 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.80 ng/mL
|
Negative CD30 expression (CD30-) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
Tras-Gly3-Vakl-Cit-PAB-PNU-159682 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.24 ng/mL
|
High HER2 expression (HER2+++; 694,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.00 ng/mL
|
Moderate HER2 expression (HER2++; 32,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
HuXBR1-402-G5-PNU [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.27 ng/mL
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell lines obtained from ATCC were serum starved for 16 hours and reintroduced to full serum media supplemented huXBR1-402-G5-PNU for 72 hours. Viability was measured by using MTS assays, and relative light unit (RLU) values for each dose were generated after 72 hours of treatment in 2 independent experiments.
|
||||
| In Vitro Model | Childhood B acute lymphoblastic leukemia | Kasumi-2 cells | CVCL_0590 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
75.68 ng/mL
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
Cell lines obtained from ATCC were serum starved for 16 hours and reintroduced to full serum media supplemented huXBR1-402-G5-PNU for 72 hours. Viability was measured by using MTS assays, and relative light unit (RLU) values for each dose were generated after 72 hours of treatment in 2 independent experiments.
|
||||
| In Vitro Model | Childhood B acute lymphoblastic leukemia | 697 cells | CVCL_0079 | ||
hCD46-29 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
41.00 pM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.60 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
hCD46-25 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
95.00 pM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
5.19 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
HuIgG1-26 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1000 pM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.10 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
HuIgG1-25 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 1000 pM | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 3.00 nM | Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
hCD46-26 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.10 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.19 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
HuIgG1-29 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.40 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Uterine sarcoma | MES-SA cells | CVCL_1404 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.00 nM
|
Positive CD46 expression (CD46 +++/++) | ||
| Method Description |
Cells were seeded at 5000 per well in a 96-well plate in complete RPMI 1640. Antibody-ZAP complexes (Advanced Targeting Systems; produced according to manufacturer's instructions) or ADCs were added to the cells and plates incubated for 72 hours and 5% carbon dioxide.
|
||||
| In Vitro Model | Normal | HEK293T cells | CVCL_0063 | ||
Brentuximab-PNUEDAGly5 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
4.80 ng/mL
|
High HER2 expression (HER2 +++) | ||
| Method Description |
For this, cells were plated on 96 well plates in 100 ul DMEM/10% FCS at adensity of 104 cells per well and assays were performed.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
11.00 ng/mL
|
Low HER2 expression (HER2 +) | ||
| Method Description |
For this, cells were plated on 96 well plates in 100 ul DMEM/10% FCS at adensity of 104 cells per well and assays were performed.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
References
