Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FPQOM
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
XMT-1660
|
|||||
| Synonyms |
XMT 1660; XMT-1660; XMT1660
Click to Show/Hide
|
|||||
| Organization |
Mersana Therapeutics, Inc.
|
|||||
| Drug Status |
Phase 1
|
|||||
| Indication |
In total 6 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
6
|
|||||
| Structure |
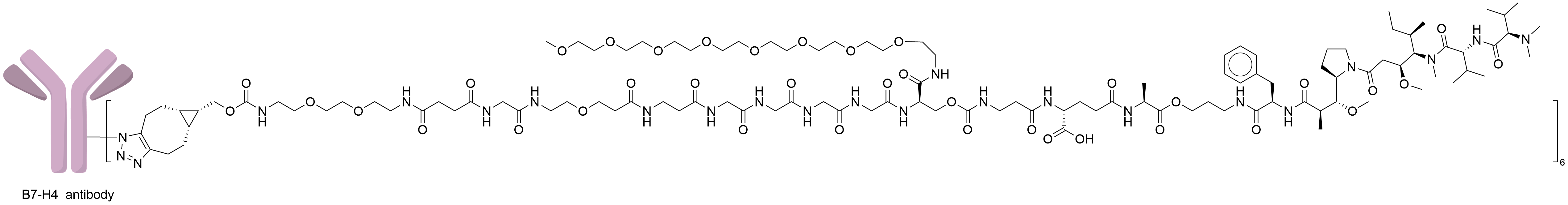
|
|||||
| Antibody Name |
Emiltatug
|
Antibody Info | ||||
| Antigen Name |
V-set domain-containing T-cell activation inhibitor 1 (VTCN1)
|
Antigen Info | ||||
| Payload Name |
Dolasynthen
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Cleavable ester linker
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
ledadotin
|
|||||
