Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ENYXZ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MRG-002
|
|||||
| Synonyms |
MRG 002; MRG-002; MRG002
Click to Show/Hide
|
|||||
| Organization |
Shanghai Miracogen Inc.; Lepu Biopharma Co., Ltd.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 7 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.8
|
|||||
| Structure |
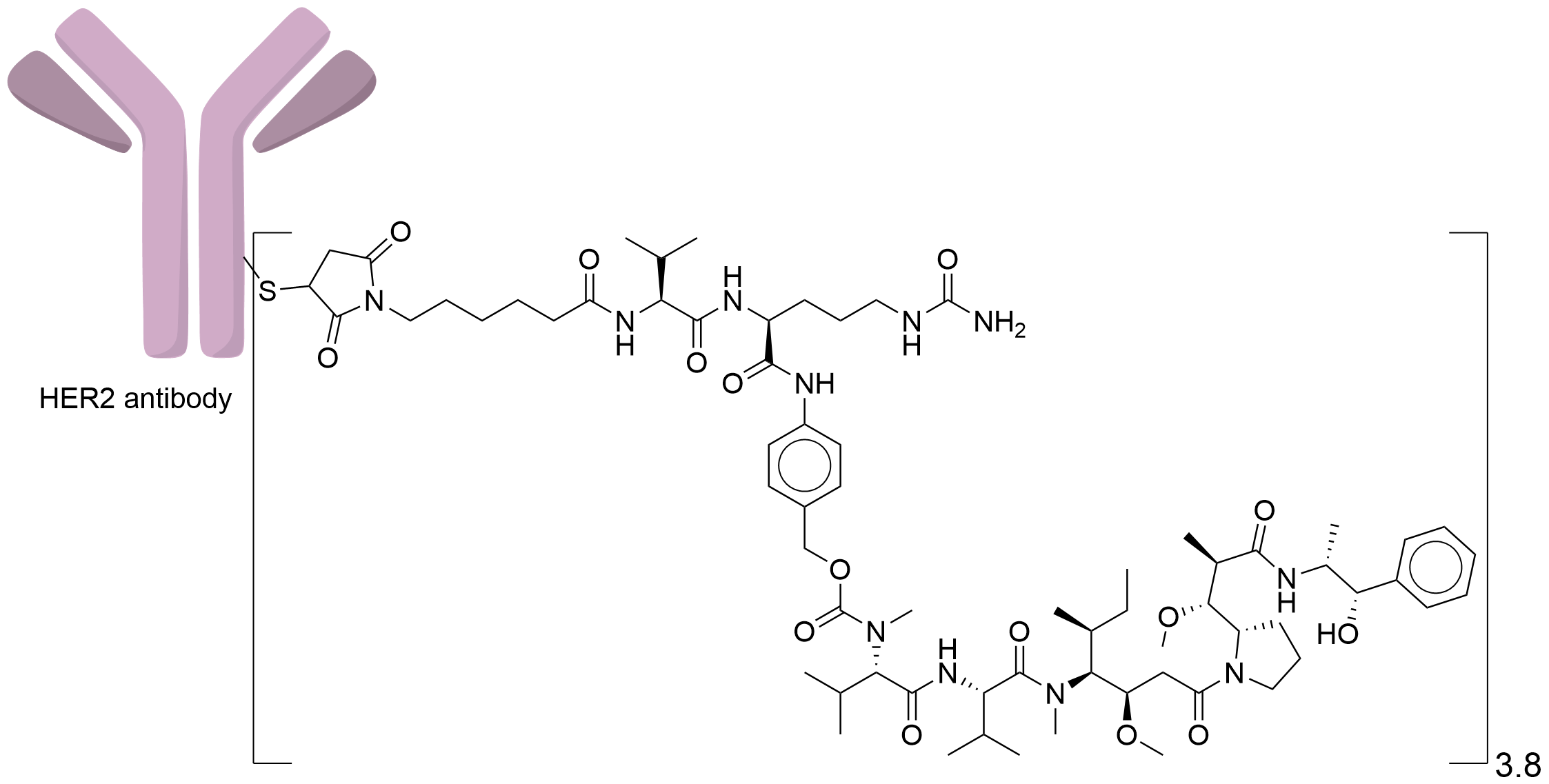
|
|||||
| Antibody Name |
MAB802
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
| Special Approval(s) |
Orphan drug(FDA)
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
34.70%
|
|||
| Patients Enrolled |
Advanced/metastatic HER2-low expressing breast cancer that failed standard therapies.
|
||||
| Administration Dosage |
MRG002 was administered intravenously once every 3 weeks at the dose of 2.60 mg/kg, until disease progression or unacceptable toxicity which ever occurred first.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04742153 | Clinical Status | Phase 2 | ||
| Clinical Description | A multicenter, non-randomized, open-label phase 2 clinical study to evaluate the efficacy and safety of MRG002 in the treatment of patients with HER2-low locally advanced or metastatic breast cancer (BC). | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
65.00%
|
|||
| Patients Enrolled |
Histologically HER2-positive (IHC 2+ or 3+) UC pts confirmed by a central-laboratory, ECOG PS 0-1, prior received 1 standard treatment.
|
||||
| Administration Dosage |
Receive MRG002 at a dose of 2.60 mg/kg or 2.20 mg/kg administered by intravenous infusion every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04839510 | Clinical Status | Phase 2 | ||
| Clinical Description | An open-label, single-arm, multi-center, phase 2 clinical study of MRG002 in the treatment of patients with HER2-positive unresectable locally advanced or metastatic urothelium cancer. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05754853 | Clinical Status | Phase 3 | ||
| Clinical Description | An open-label, randomized, multi-center, phase 3 clinical study of MRG002 versus investigator's choice of chemotherapy in the treatment of patients with HER2-positive unresectable locally advanced or metastatic urothelial cancer previously treated with platinum-based chemotherapy and PD-1/PD-L1 inhibitors. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04924699 | Clinical Status | Phase 2/3 | ||
| Clinical Description | A study of MRG002 in the treatment of patients with HER2-positive unresectable locally advanced or metastatic breast cancer. | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05263869 | Clinical Status | Phase 2 | ||
| Clinical Description | An open-label, multi-center, single-arm phase 2 clinical study to evaluate the efficacy and safety of MRG002 in advanced HER-2 positive breast cancer patients previously treated with trastuzumab and TKIs (Magic-009). | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05141786 | Clinical Status | Phase 2 | ||
| Clinical Description | An open-label, multi-center, non-randomized phase 2 clinical study to evaluate the efficacy and safety of MRG002 in patients With HER2-mutated unresectable/metastatic non-small cell lung cancer (NSCLC). | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05141747 | Clinical Status | Phase 2 | ||
| Clinical Description | An open-label, multi-center, phase 2 clinical study to evaluate the safety, efficacy and pharmacokinetics of MRG002 in patients with HER2-positive/HER2-low locally advanced or metastatic gastric/ gastroesophageal junction cancer. | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04837508 | Clinical Status | Phase 2 | ||
| Clinical Description | An open-label, single-arm, multi-center, phase 2 clinical study of MRG002 in the treatment of patients with HER2-positive unresectable, locally advanced or metastatic biliary tract cancer. | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [9] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05338957 | Clinical Status | Phase 1/2 | ||
| Clinical Description | An open-label, multi-center, phase 1/2 dose escalation and expansion study to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of MRG002 in combination with HX008 in patients with HER2-expressed advanced malignant solid tumors. | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [10] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04492488 | Clinical Status | Phase 1/2 | ||
| Clinical Description | An open-label, multi-center phase 1/2 dose escalation and expansion study to assess the safety, efficacy and pharmacokinetics of MRG002 in patients with HER2-positive advanced solid tumors and locally advanced or metastatic gastric/gastroesophageal junction (GEJ) cancer. | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [11] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04941339 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label, multi-center, first in human, dose escalation and expansion study to assess the safety, tolerability, efficacy and pharmacokinetics of MRG002 in patients with HER2 positive advanced solid tumors. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 0.00% (Day 21) | Moderate HER2 expression (HER2++; IHC 2+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#151) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 10.00% (Day 21) | Low HER2 expression (HER2+; IHC 1+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#395) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 17.00% (Day 21) | High HER2 expression (HER2+++; IHC 3+) | ||
| In Vivo Model | HER2-positive breast cancer PDX model (PDX: BC#239) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 19.00% (Day 21) | Moderate HER2 expression (HER2++; IHC 2+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#053) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 41.00% (Day 21) | Moderate HER2 expression (HER2++; IHC 2+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#240) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.10% (Day 63) | High HER2 expression (HER2+++; IHC 3+) | ||
| In Vivo Model | HER2-positive breast cancer PDX model (PDX: BC#046) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.00% (Day 56) | Moderate HER2 expression (HER2++; IHC 2+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#179) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 70.40% (Day 35) | High HER2 expression (HER2+++; IHC 3+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#410) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.90% (Day 24) | |||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#410) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
84.00% (Day 21)
|
|||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#410) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.90% (Day 70) | |||
| In Vivo Model | HER2-positive breast cancer PDX model (PDX: BC#197) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
90.00% (Day 21)
|
|||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#069) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.00% (Day 63) | |||
| In Vivo Model | HER2-positive breast cancer PDX model (PDX: BC#046) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.10% (Day 56) | |||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#179) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 70) | High HER2 expression (HER2+++; IHC 3+) | ||
| In Vivo Model | HER2-positive breast cancer PDX model (PDX: BC#197) | ||||
| Experiment 16 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 35) | Low HER2 expression (HER2+; IHC 1+) | ||
| In Vivo Model | HER2-positive gastric cancer PDX model (PDX: STO#410) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.70% (Day 36) | Moderate HER2 expression (HER2++) | ||
| In Vivo Model | HER2-positive gastric cancer NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.20% (Day 36) | High HER2 expression (HER2+++) | ||
| Method Description |
MRG-002 induces efficient tumor cell killing in PDX models of breast cancer or gastric cancer tissues with HER2 expression,administered with vehicle,MRG002,HX008 or MRG002 + HX008 combo intravenously.
|
||||
| In Vivo Model | HER2-positive breast cancer BT-474 CDX model | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 36) | Moderate HER2 expression (HER2++; IHC 2+) | ||
| In Vivo Model | HER2-positive breast cancer BT-474 CDX model | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 36) | Moderate HER2 expression (HER2++; IHC 2+) | ||
| In Vivo Model | HER2-positive breast cancer BT-474 CDX model | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 36) | High HER2 expression (HER2+++) | ||
| In Vivo Model | HER2-positive gastric cancer NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.00% (Day 36) | High HER2 expression (HER2+++) | ||
| In Vivo Model | HER2-positive gastric cancer NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
|||
| Method Description |
The inhibitory activity of MRG-002 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with MRG-002 for 96±2 hrs.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
|||
| Method Description |
The inhibitory activity of MRG-002 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with MRG-002 for 96±2 hrs.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
|||
| Method Description |
The inhibitory activity of MRG-002 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with MRG-002 for 96±2 hrs.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.40 nM
|
|||
| Method Description |
The inhibitory activity of MRG-002 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated with MRG-002 for 96±2 hrs.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
References
