Linker Information
General Information of This Linker
| Linker ID |
LIN0JWFUO
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
MCC-Gly6-Thr-Glu3-Pro-Leu-Ala3-Leu
|
|||||
| Linker Type |
Flexible reactive (thiol) linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
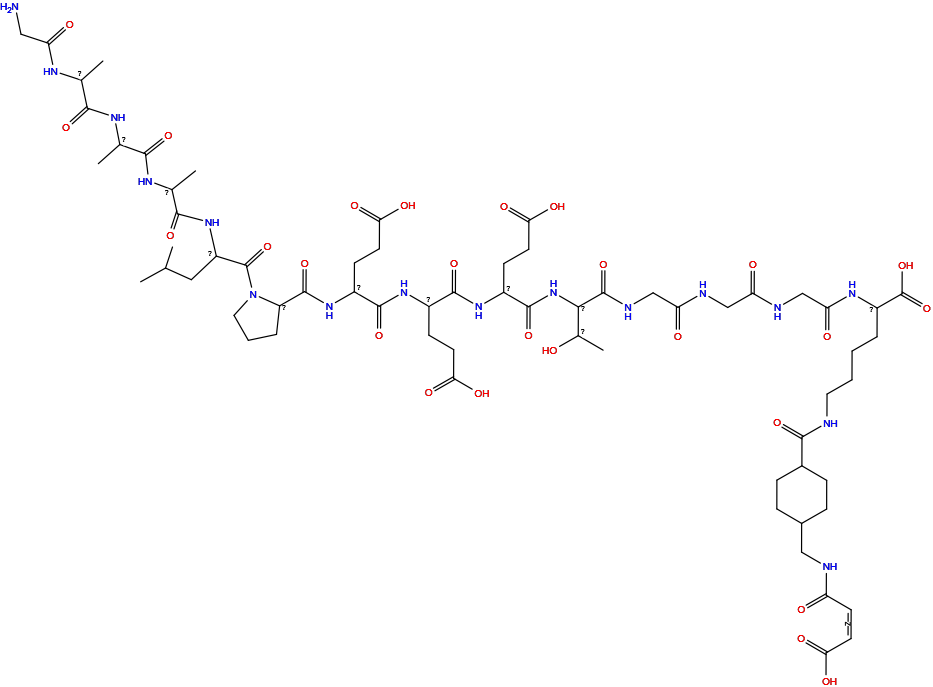
|
|||||
| Formula |
C65H102N16O26
|
|||||
| Isosmiles |
CC(C)CC(NC(=O)C(C)NC(=O)C(C)NC(=O)C(C)NC(=O)CN)C(=O)N1CCCC1C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(C(=O)NCC(=O)NCC(=O)NCC(=O)NC(CCCCNC(=O)C1CCC(CNC(=O)/C=C\C(=O)O)CC1)C(=O)O)C(C)O
|
|||||
| InChI |
InChI=1S/C65H102N16O26/c1-32(2)26-43(79-57(98)35(5)74-56(97)34(4)73-55(96)33(3)72-46(84)27-66)64(105)81-25-9-11-44(81)62(103)78-40(17-21-51(90)91)60(101)76-39(16-20-50(88)89)59(100)77-41(18-22-52(92)93)61(102)80-54(36(6)82)63(104)71-30-48(86)69-29-47(85)70-31-49(87)75-42(65(106)107)10-7-8-24-67-58(99)38-14-12-37(13-15-38)28-68-45(83)19-23-53(94)95/h19,23,32-44,54,82H,7-18,20-22,24-31,66H2,1-6H3,(H,67,99)(H,68,83)(H,69,86)(H,70,85)(H,71,104)(H,72,84)(H,73,96)(H,74,97)(H,75,87)(H,76,101)(H,77,100)(H,78,103)(H,79,98)(H,80,102)(H,88,89)(H,90,91)(H,92,93)(H,94,95)(H,106,107)/b23-19-
|
|||||
| InChIKey |
ZUGYJLXBYFMVJK-NMWGTECJSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
1523.617
|
Polar area
|
660.46
|
||
|
Complexity
|
1522.715117
|
xlogp Value
|
-7.3366
|
|||
|
Heavy Count
|
107
|
Rot Bonds
|
48
|
|||
|
Hbond acc
|
22
|
Hbond Donor
|
21
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
GQ-1001 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
40.00%
|
|||
| Patients Enrolled |
HER2-positive advanced solid tumors.
|
||||
| Administration Dosage |
Administered intravenously as a monotherapy on Day 1 of 21-day cycles. The starting dose was 1.20 mg/kg, followed by 2.40, 3.60, 4.80, 6.00, 7.20 and 8.40 mg/kg.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04450732 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1, first-in-human, multicenter, open-label, study of GQ1001, a HER2 targeted antibody-drug conjugate, administered intravenously, in adult patients with HER2-positive advanced solid tumors.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05575804 | Clinical Status | Phase 1/2 | ||
| Clinical Description |
Phase 1b/2 study of GQ1001 and pyrotinib in HER2 positive metastatic breast cancer patients who had failed previous anti-HER2 treatment GRACE.
|
||||
References
