Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0AZJPR
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Magacizumab PD-MMAE ADC 5
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
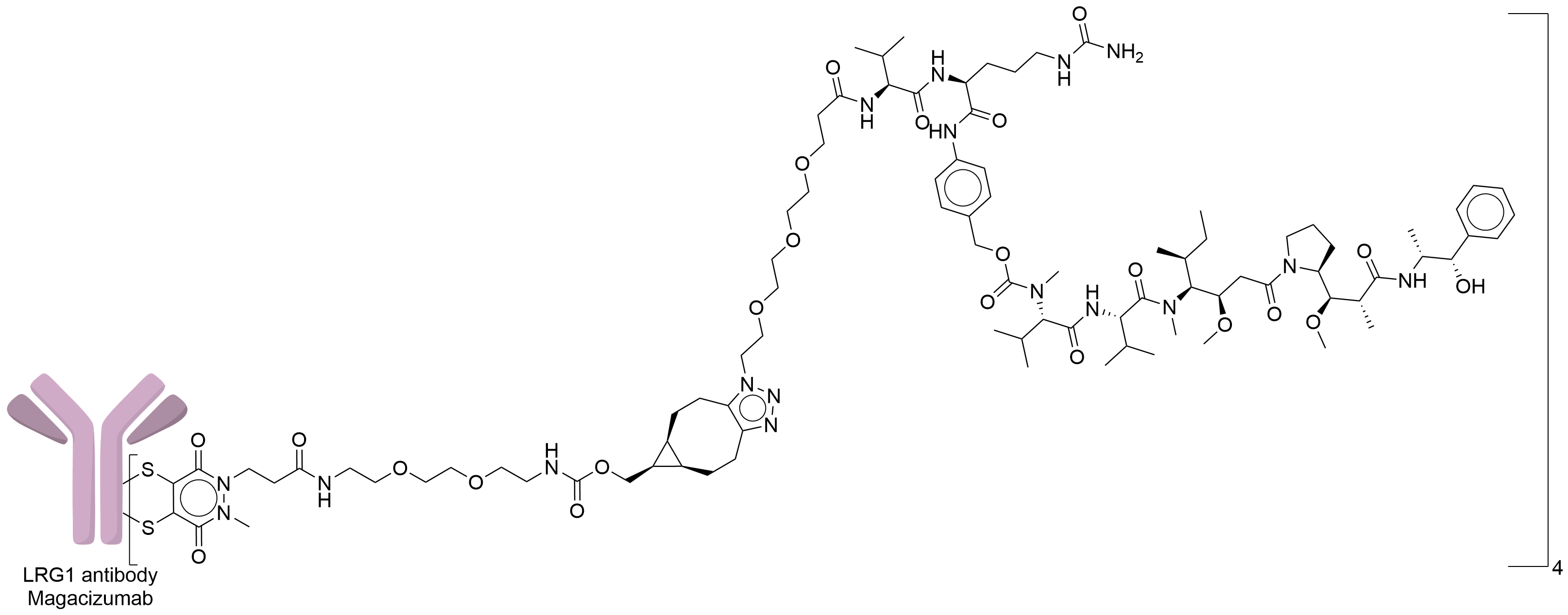
|
|||||
| Antibody Name |
Magacizumab
|
Antibody Info | ||||
| Antigen Name |
Leucine-rich alpha-2-glycoprotein (LRG1)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mepstra-PD-PEG3-Mal-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Pyridazinediones (PDs) was applied to functionally rebridge cysteine residues liberated upon reduction of interchain disulfide bonds as the antibody conjugation method.
|
|||||
