Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0AJQLB
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ADC HE-S2
|
|||||
| Synonyms |
ADC PD-L1 HE-S2
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
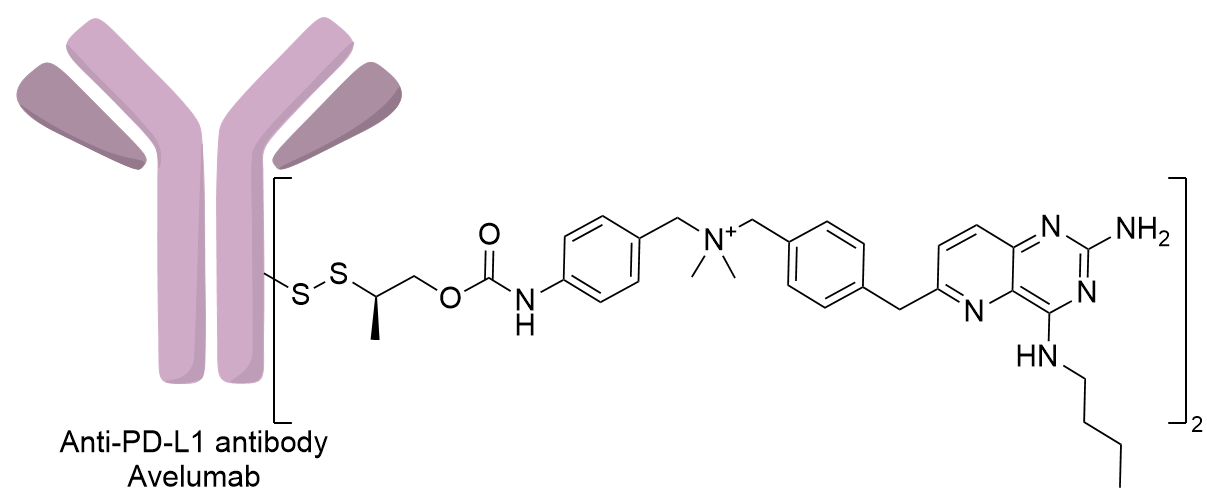
|
|||||
| Antibody Name |
Avelumab
|
Antibody Info | ||||
| Antigen Name |
Programmed cell death 1 ligand 1 (CD274)
|
Antigen Info | ||||
| Payload Name |
Bifunctional immunomodulator D18
|
Payload Info | ||||
| Therapeutic Target |
Toll-like receptor 7 (TLR7); Toll-like receptor 8 (TLR8)
|
Target Info | ||||
| Linker Name |
Disulfide linker 8
|
Linker Info | ||||
| Conjugate Type |
Avelumab was mutated at light-chain V205 position to generate an engineered anti-PD-L1 antibody with two accessible free cysteine, Engineered reactive cysteine residues (THIOMAB) strategy for site-specific conjugation.
|
|||||
| Combination Type |
HE-S2
|
|||||
