Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0AFXBF
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Cetuximab-Puromycin
|
|||||
| Synonyms |
Cetuximab-SATP-Puromycin
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
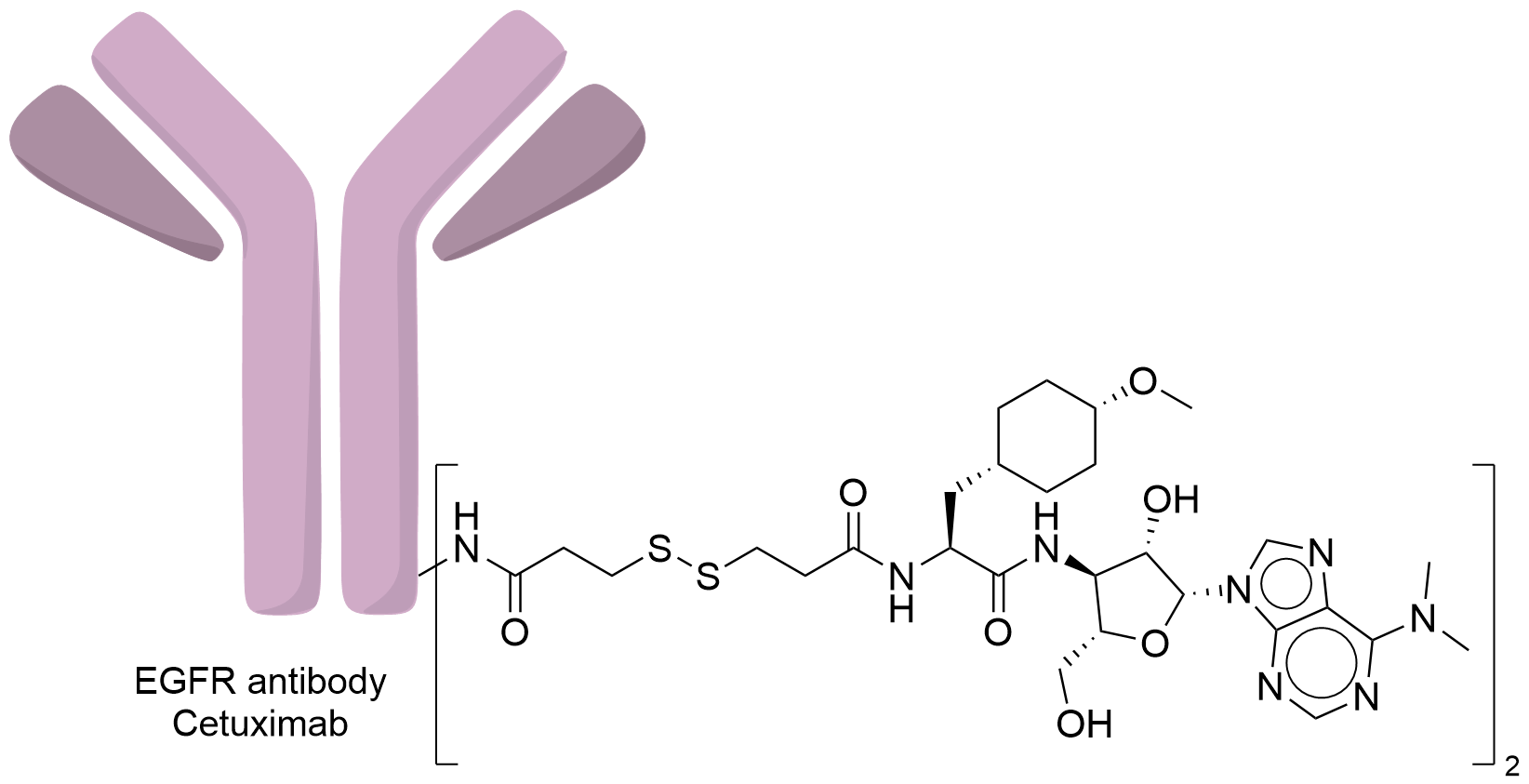
|
|||||
| Antibody Name |
Cetuximab
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
Puromycin
|
Payload Info | ||||
| Therapeutic Target |
Ribosome (RB)
|
Target Info | ||||
| Linker Name |
Succinimidyl acetylthiopropionate (SATP)
|
Linker Info | ||||
| Conjugate Type |
Puromycin was conjugated to Cetuximab by forming disulfide bonds between thiolated Cetuximab and thiolated Puromycin.
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 37.42 ug/mL | High EGFR expression (EGFR+++) | ||
| Method Description |
Cells were seeded into 6-well plates, 500,000 cells/well. One day (24 hours) before treatment, MDA-MB-231 cells are being fasted from FBS. Cells are divided into five different doses of conjugate (5 ug/mL; 10 ug/mL; 20 ug/mL; 40 ug/mL; 80 ug/mL) and one untreated group. They were incubated for 48 hours.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
