Payload Information
General Information of This Payload
| Payload ID | PAY0OWBST |
|||||
|---|---|---|---|---|---|---|
| Name | Puromycin |
|||||
| Synonyms |
Puromycin; 53-79-2; Stylomycin; Puromycine; Puromicina; Puromycinum; P-638; 3123L; Puromycin dihydrochloride; CHEBI:17939; GNF-PF-2016; CL 13,900; Stillomycin; CL 13900; CL-13900; Puromycine [INN-French]; Puromycinum [INN-Latin]; Puromicina [INN-Spanish]; UNII-4A6ZS6Q2CL; 4A6ZS6Q2CL; 3'-(L-alpha-Amino-p-methoxyhydrocinnamamido)-3'-deoxy-N,N-dimethyladenosine; Puromycin [USAN:INN:BAN]; (S)-3'-((2-Amino-3-(4-methoxyphenyl)-1-oxopropyl)amino)-3'-deoxy-N,N-dimethyladenosine; P 638; Puromycin (USAN); PUROMYCIN [USAN]; NSC 3055; 3123-L; 9-{3-deoxy-3-[(O-methyl-L-tyrosyl)amino]-beta-D-xylofuranosyl}-N,N-dimethyl-9H-purin-6-amine; Adenosine, 3'-(((2S)-2-amino-3-(4-methoxyphenyl)-1-oxopropyl)amino)-3'-deoxy-N,N-dimethyl-; (S)-2-amino-N-((2S,3S,4R,5R)-5-(6-(dimethylamino)-9H-purin-9-yl)-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl)-3-(4-methoxyphenyl)propanamide; Achromycin (purine derivative); TCMDC-123493; 3'-deoxy-N,N-dimethyl-3'-(O-methyl-L-tyrosinamido)adenosine; C22H29N7O5; 3'-[[(2S)-2-amino-3-(4-methoxyphenyl)-1-oxopropyl]amino]-3'-deoxy-N,N-diemthyladenosine; 6-Dimethylamino-9-(3'-(p-methoxy-L-phenylalanylamino)-beta-D-ribofuranosyl)-purine; CL 16536; NSC3055; NSC-3055; Adenosine, 3'-((2-amino-3-(4-methoxyphenyl)-1-oxopropyl)amino)-3'-deoxy-N,N-dimethyl-, (S)-; 58-58-2; 3'-deoxy-N,N-dimethyl-3'-[(O-methyl-L-tyrosyl)amino]adenosine; (S)-3'-[[2-Amino-3-(4-methoxyphenyl)-1-oxopropyl]amino]-3'-deoxy-N,N-dimethyladenosine; Adenosine, 3'-[[(2S)-2-amino-3-(4-methoxyphenyl)-1-oxopropyl]amino]-3'-deoxy-N,N-dimethyl-; PUY; PUROMYCIN [INN]; PUROMYCIN [MI]; Prestwick3_000480; Epitope ID:140945; PUROMYCIN [WHO-DD]; SCHEMBL4570; BSPBio_000620; Puromycin [USAN:BAN:INN]; BPBio1_000682; CHEMBL469912; DTXSID8036788; 3'-(L-.alpha.-Amino-p-methoxyhydrocinnamamido)-3'-deoxy-N,N-dimethyladenosine; Adenosine, 3'-(alpha-amino-p-methoxyhydrocinnamamido)-3'-deoxy-N,N-dimethyl-, L-; RXWNCPJZOCPEPQ-NVWDDTSBSA-N; HMS2089F18; HY-B1743; CL 16,536 (*Dihydrochloride*); BDBM50277158; MFCD00866328; AKOS030485964; C22-H29-N7-O5; CCG-208505; CS-6500; DB08437; Puromycin, 10 mg/ml in distilled water; NCGC00179500-01; NCGC00179500-07; NCGC00179500-14; (2S)-2-amino-N-[(2S,3S,4R,5R)-5-[6-(dimethylamino)purin-9-yl]-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]-3-(4-methoxyphenyl)propanamide; AB00514661; C01610; D05653; AB00514661-07; Q424696; BRD-K36007650-001-01-9; BRD-K36007650-300-02-3; Q27167243; L-3'-(alpha-amino-p-methoxyhydrocinnamamido)-3'-deoxy-N,N-dimethyladenosine; (2S)-2-amino-N-[(2S,3S,4R,5R)-5-[6-(dimethylamino)purin-9-yl]-4-hydroxy-2-(hydroxymethyl)tetrahydrofuran-3-yl]-3-(4-methoxyphenyl)propanamide; 2-amino-N-[5-[6-(dimethylamino)-9-purinyl]-4-hydroxy-2-(hydroxymethyl)-3-oxolanyl]-3-(4-methoxyphenyl)propanamide; Adenosine, 3'-(-amino-p-methoxyhydrocinnamamido)-3'-deoxy-N, N-dimethyl-, dihydrochloride, L-
Click to Show/Hide
|
|||||
| Target(s) | Ribosome (RB) | |||||
| Structure |
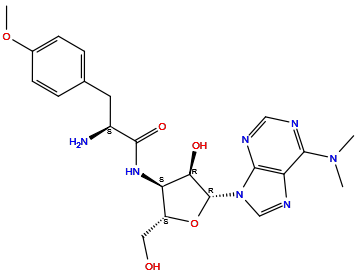
|
|||||
| Formula | C22H29N7O5 |
|||||
| Isosmiles | CN(C)C1=NC=NC2=C1N=CN2[C@H]3[C@@H]([C@@H]([C@H](O3)CO)NC(=O)[C@H](CC4=CC=C(C=C4)OC)N)O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C22H29N7O5/c1-28(2)19-17-20(25-10-24-19)29(11-26-17)22-18(31)16(15(9-30)34-22)27-21(32)14(23)8-12-4-6-13(33-3)7-5-12/h4-7,10-11,14-16,18,22,30-31H,8-9,23H2,1-3H3,(H,27,32)/t14-,15+,16+,18+,22+/m0/s1
|
|||||
| InChIKey |
RXWNCPJZOCPEPQ-NVWDDTSBSA-N
|
|||||
| IUPAC Name |
(2S)-2-amino-N-[(2S,3S,4R,5R)-5-[6-(dimethylamino)purin-9-yl]-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]-3-(4-methoxyphenyl)propanamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
471.5 |
Polar area |
161 |
||
Complexity |
680 |
xlogp Value |
0 |
|||
Heavy Count |
34 |
Rot Bonds |
8 |
|||
Hbond acc |
10 |
Hbond Donor |
4 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.5 | ug/mL |
P388 cells
|
Lymphoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.35 | nM |
HEK293 cells
|
Normal
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10000 | nM |
J774.A1 cells
|
Mouse reticulum cell sarcoma
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 170 | nM |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 20200 | nM |
HepG2/Adm cells
|
Hepatoblastoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 210 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 210 | nM |
KB 3-1 cells
|
Cervical epithelial carcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 220 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 230 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 26830 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 300 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 300 | nM |
PC-3 cells
|
Prostate carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 350.5 | nM |
HEK293 cells
|
Normal
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 360 | nM |
HEK293 cells
|
Normal
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 360 | nM |
HEK293 cells
|
Normal
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 361 | nM |
HEK293 cells
|
Normal
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 399 | nM |
HEK293 cells
|
Normal
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 400 | nM |
HEK293 cells
|
Normal
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 420 | nM |
HEK293 cells
|
Normal
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 450 | nM |
HEK293 cells
|
Normal
|
[15] | |
| Half Maximal Inhibitory Concentration (IC50) | 460 | nM |
HEK293 cells
|
Normal
|
[16] | |
| Half Maximal Inhibitory Concentration (IC50) | 460 | nM |
HEK293 cells
|
Normal
|
[17] | |
| Half Maximal Inhibitory Concentration (IC50) | 5 | nM |
WI-38 VA13 cells
|
Normal
|
[18] | |
| Half Maximal Effective Concentration (EC50) | 55 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 600 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[19] | |
| Half Maximal Effective Concentration (EC50) | 6100 | nM |
HCT-8 cells
|
Ileocecal adenocarcinoma
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | 694 | nM |
HEK293 cells
|
Normal
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 700 | nM |
PC-3 cells
|
Prostate carcinoma
|
[19] | |
| Half Maximal Inhibitory Concentration (IC50) | 79300 | nM |
KB-V1 cells
|
Cervical carcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 810 | nM |
HEK293 cells
|
Normal
|
[22] | |
| Half Maximal Inhibitory Concentration (IC50) | 83930 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[6] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Cetuximab-Puromycin [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [23] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
37.42 ug/mL
|
High EGFR expression (EGFR+++) | ||
| Method Description |
Cells were seeded into 6-well plates, 500,000 cells/well. One day (24 hours) before treatment, MDA-MB-231 cells are being fasted from FBS. Cells are divided into five different doses of conjugate (5 ug/mL; 10 ug/mL; 20 ug/mL; 40 ug/mL; 80 ug/mL) and one untreated group. They were incubated for 48 hours.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
