Payload Information
General Information of This Payload
| Payload ID | PAY0VDATG |
|||||
|---|---|---|---|---|---|---|
| Name | SG2000 |
|||||
| Synonyms |
SJG-136; 232931-57-6; SJG 136; NSC-694501; SG-2000; BN-2629; UNII-KT0ZQ64X1A; NSC 694501; UP 2001; KT0ZQ64X1A; SJG136; (6aS)-3-[3-[[(6aS)-2-methoxy-8-methylidene-11-oxo-7,9-dihydro-6aH-pyrrolo[2,1-c][1,4]benzodiazepin-3-yl]oxy]propoxy]-2-methoxy-8-methylidene-7,9-dihydro-6aH-pyrrolo[2,1-c][1,4]benzodiazepin-11-one; SP-2001; UP-2001; 5H-Pyrrolo[2,1-c][1,4]benzodiazepin-5-one, 8,8'-[1,3-propanediylbis(oxy)]bis[1,2,3,11a-tetrahydro-7-methoxy-2-methylene-, (11aS,11'aS)-; 5H-PYRROLO(2,1-C)(1,4)BENZODIAZEPIN-5-ONE, 8,8'-(1,3-PROPANEDIYLBIS(OXY))BIS(1,2,3,11A-TETRAHYDRO-7-METHOXY-2-METHYLENE-, (11AS,11'AS)-; NSC694501; CHEMBL16498; SCHEMBL12020905; SJG 136 [WHO-DD]; DTXSID20177864; CS-4593; DB11965; BS-42683; HY-14573; NCI60_033825; C74198; A858346; Q27282424; (11AS,11a'S)-8,8'-(propane-1,3-diylbis(oxy))bis(7-methoxy-2-methylene-1,2,3,11a-tetrahydro-5H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-5-one); (11aS,11a'S)-8,8'-(propane-1,3-diylbis(oxy))bis(7-methoxy-2-methylene-1,2,3,11a-tetrahydro-5H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-5-one);1,1'-(propane-1,3-diylbis(oxy))bis(7-methoxy-2-methylene-1,2,3,11a-tetrahydro-5H-benzo[e]pyrrolo[1,2-a][1,4]diazepine-5,11(10H)-dione); (6AS)-3-(3-(((6AS)-2-METHOXY-8-METHYLENE-11-OXO-7,9-DIHYDRO-6AH-PYRROLO(2,1-C)(1,4)BENZODIAZEPIN-3-YL)OXY)PROPOXY)-2-METHOXY-8-METHYLENE-7,9-DIHYDRO-6AH-PYRROLO(2,1-C)(1,4)BENZODIAZEPIN-11-ONE; 1,1'-((Propane-1,3-diyl)dioxy)bis(7-methoxy-2-methylidene-1,2,3,10,11,11a-hexahydro-5H-pyrrolo(2,1-c)(1,4)benzodiazepin-5,11-dione); 5H-Pyrrolo[2,4]benzodiazepin-5-one, 8,8'-[1,3-propanediylbis(oxy)]bis[1,2,3,11a-tetrahydro- 7-methoxy-2-methylene-, (11aS, 11'aS)-
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
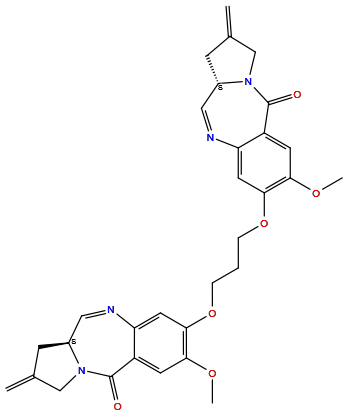
|
|||||
| Formula | C31H32N4O6 |
|||||
| Isosmiles | COC1=C(C=C2C(=C1)C(=O)N3CC(=C)C[C@H]3C=N2)OCCCOC4=C(C=C5C(=C4)N=C[C@@H]6CC(=C)CN6C5=O)OC |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C31H32N4O6/c1-18-8-20-14-32-24-12-28(26(38-3)10-22(24)30(36)34(20)16-18)40-6-5-7-41-29-13-25-23(11-27(29)39-4)31(37)35-17-19(2)9-21(35)15-33-25/h10-15,20-21H,1-2,5-9,16-17H2,3-4H3/t20-,21-/m0/s1
|
|||||
| InChIKey |
RWZVMMQNDHPRQD-SFTDATJTSA-N
|
|||||
| IUPAC Name |
(6aS)-3-[3-[[(6aS)-2-methoxy-8-methylidene-11-oxo-7,9-dihydro-6aH-pyrrolo[2,1-c][1,4]benzodiazepin-3-yl]oxy]propoxy]-2-methoxy-8-methylidene-7,9-dihydro-6aH-pyrrolo[2,1-c][1,4]benzodiazepin-11-one
|
|||||
| Pharmaceutical Properties | Molecule Weight |
556.6 |
Polar area |
102 |
||
Complexity |
1020 |
xlogp Value |
1.9 |
|||
Heavy Count |
41 |
Rot Bonds |
8 |
|||
Hbond acc |
8 |
Hbond Donor |
0 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.0225 | nM |
A2780 cells
|
Ovarian endometrioid adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.0225 | nM |
A2780 cells
|
Ovarian endometrioid adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.024 | nM |
A2780 cells
|
Ovarian endometrioid adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.12 | nM |
CH1 cells
|
Ovarian carcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.1361 | nM |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.8318 | nM |
SF268 cells
|
Astrocytoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.4 | nM |
NCI-H522 cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.66 | nM |
NCI-H23 cells
|
Lung adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SN12C cells
|
Renal cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
U-251MG cells
|
Astrocytoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
CCRF-CEM cells
|
T acute lymphoblastic leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
COLO 205 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
Caki-1 cells
|
Clear cell renal cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SK-MEL-5 cells
|
Cutaneous melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SNB-19 cells
|
Astrocytoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
T-47D cells
|
Invasive breast carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
786-O cells
|
Renal cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
IGROV-1 cells
|
Ovarian endometrioid adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SF539 cells
|
Gliosarcoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SNB-75 cells
|
Glioblastoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
ACHN cells
|
Renal adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
LOX IMVI cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SF-295 cells
|
Glioblastoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SR cells
|
Leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
UACC-62 cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.33 | nM |
HCT 116 cells
|
Colon carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.64 | nM |
HOP-62 cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.67 | nM |
RXF 393 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.76 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.91 | nM |
Malme-3M cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 11.12 | nM |
BT-549 cells
|
Breast ductal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 11.86 | nM |
OVCAR-8 cells
|
High grade ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.02 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.11 | nM |
RPMI-8226 cells
|
Plasma cell myeloma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.25 | nM |
NCI-H226 cells
|
Pleural epithelioid mesothelioma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.3 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.39 | nM |
KM12 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.56 | nM |
HCC 2998 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 123.59 | nM |
HCT 15 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.06 | nM |
SK-MEL-28 cells (BRAF inhibitor resistant)
|
Cutaneous melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.29 | nM |
MDA-N cells
|
Breast carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.55 | nM |
SK-MEL-2 cells (MEK inhibitor-resistant)
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.62 | nM |
A498 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.62 | nM |
UACC-257 cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.69 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 15.7 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 16.9 | nM |
M14 cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 17.18 | nM |
OVCAR-3 cells (FZD7 overexpression)
|
Ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 17.74 | nM |
DU145 cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 170 | nM |
CHO cells
|
Normal
|
[4] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 18.07 | nM |
MDA-MB-435 cells
|
Amelanotic melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 19.05 | nM |
PC-3 cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 20.84 | nM |
UO-31 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 200 | nM |
OE33 cells
|
Barrett adenocarcinoma
|
[5] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 21.58 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 29.31 | nM |
OVCAR-5 cells
|
Ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 29.92 | nM |
NCI-H322M cells
|
Minimally invasive lung adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 30.55 | nM |
OVCAR-4 cells
|
Ovarian adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 31.62 | nM |
TK-10 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 32.73 | nM |
EKVX cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 42.5 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 42.56 | nM |
Hs 578T cells
|
Invasive breast carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 43 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[6] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.823 | nM |
HOP-92 cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 8 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[7] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 83.37 | nM |
NCI-ADR-RES cells
|
High grade ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 9.1 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[2] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
WO2021044208A1 ADC6 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% (Day 41) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line HCC1187 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 110 mm3 on average (Day 1), and 1.25 mg/kg of ADC5.
|
||||
| In Vivo Model | HCC1187 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1187 cells | CVCL_1247 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.74% (Day 26) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing lung cancer cell line Calu-3 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 111mm3 on average (Day 1), and 1 mg/kg QDx1 of ADC5.
|
||||
| In Vivo Model | Calu-3 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.97% (Day 19) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line MDA-MB231 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 110 mm3 on average (Day 1), and 0.33 mg/kg ADC5 was intravenously injected twice weekly a total of three times (Days 1, 7 and 14).
Click to Show/Hide
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.51% (Day 19) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line MDA-MB231 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 110 mm3 on average (Day 1), and 0.5 mg/kg of ADC5.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 83.90% (Day 19) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line MDA-MB231 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 110 mm3 on average (Day 1), and 1 mg/kg of ADC5.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.95% (Day 35) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing lung cancer cell line Calu-3 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 184mm3 on average (Day 1), and 1 mg/kg QDx1 of ADC5.
|
||||
| In Vivo Model | Calu-3 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.09% (Day 33) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing mantle cell lymphoma cell line JeKo-1 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 184mm3 on average (Day 1), and 0.25 mg/kg QWx4 of ADC5.
|
||||
| In Vivo Model | JeKo-1 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.16% (Day 41) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line HCC1187 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 110 mm3 on average (Day 1), and 3.75 mg/kg QWx4 of ADC2.
|
||||
| In Vivo Model | HCC1187 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | HCC1187 cells | CVCL_1247 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.98% (Day 19) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line MDA-MB231 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 110 mm3 on average (Day 1), and 2 mg/kg of ADC5.
|
||||
| In Vivo Model | MDA-MB-231 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.97% (Day 35) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing lung cancer cell line Calu-3 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 184mm3 on average (Day 1), and 0.25 mg/kg QWx4 of ADC5.
|
||||
| In Vivo Model | Calu-3 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 98.79% (Day 56) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing breast cancer cell line MDA-MB-468 were grafted to female Balb/C nude mice to prepare human cancer grafted mice. After grafting.the mice were grouped when tumor size reached 166mm3 on average (Day 1), and 1 mg/kg of ADC5 was intravenously injected into the mice. In the control group, 10ml/kg PBS was intravenously injected into the mice.
Click to Show/Hide
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.97% (Day 26) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing lung cancer cell line Calu-3 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 111mm3 on average (Day 1), and 4 mg/kg of ADC5.
|
||||
| In Vivo Model | Calu-3 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 33) | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
1 x 107 cells/head of human ROR-1 expressing mantle cell lymphoma cell line JeKo-1 were grafted to severe combined immunodeficient (SCID) mice to prepare human cancergrafted mice. After grafting, the mice were grouped when tumor size reached 184mm3 on average (Day 1), and 1 mg/kg QDx1 of ADC5.
|
||||
| In Vivo Model | JeKo-1 CDX model | ||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.09 nM
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
In a 96-well plate, each well was seeded with 4,000 to 5,000 of the respective cancer cell lines. After culturing for 24 hours, they were treated with the ADCs at a concentra-tion of 0.0015 to 10.0 nM (serially diluted threefold). 72 hours later, the number of live cells was measured using WST-8.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
In a 96-well plate, each well was seeded with 4,000 to 5,000 of the respective cancer cell lines. After culturing for 24 hours, they were treated with the ADCs at a concentra-tion of 0.0015 to 10.0 nM (serially diluted threefold). 72 hours later, the number of live cells was measured using WST-8.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2228 cells | CVCL_1543 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
In a 96-well plate, each well was seeded with 4,000 to 5,000 of the respective cancer cell lines. After culturing for 24 hours, they were treated with the ADCs at a concentra-tion of 0.0015 to 10.0 nM (serially diluted threefold). 72 hours later, the number of live cells was measured using WST-8.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
243 nM
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
In a 96-well plate, each well was seeded with 4,000 to 5,000 of the respective cancer cell lines. After culturing for 24 hours, they were treated with the ADCs at a concentra-tion of 0.0015 to 10.0 nM (serially diluted threefold). 72 hours later, the number of live cells was measured using WST-8.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
247 nM
|
Positive ROR1 expression (ROR1+++/++) | ||
| Method Description |
In a 96-well plate, each well was seeded with 4,000 to 5,000 of the respective cancer cell lines. After culturing for 24 hours, they were treated with the ADCs at a concentra-tion of 0.0015 to 10.0 nM (serially diluted threefold). 72 hours later, the number of live cells was measured using WST-8.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
OHPAS ADC-2 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 37.00% (Day 78) | High HER2 expression (HER2 +++) | ||
| Method Description |
We also tested the OHPAS ADCs in in vivo xenograft mouse models using N87 cell lines. The experiment was followed up to 110 days after administration of ADCs at two different doses (0.5 mg/kg) on day one (initial tumor volume 100 mm3).
|
||||
| In Vivo Model | NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.60% (Day 78) | High HER2 expression (HER2 +++) | ||
| Method Description |
We also tested the OHPAS ADCs in in vivo xenograft mouse models using N87 cell lines. The experiment was followed up to 110 days after administration of ADCs at two different doses (2 mg/kg) on day one (initial tumor volume 100 mm3).
|
||||
| In Vivo Model | NCI-N87 CDX model | ||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
High HER2 expression (HER2 +++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08 nM
|
Moderate HER2 expression (HER2 ++) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 50.00 nM | Negative HER2 expression (HER2 -) | ||
| Method Description |
With OHPAS ADCs 1-4 , we first performed cell-based MTT assays against a series of HER2 positive/negative cell lines using the commercially available HER2 ADC, T-DM1 (average DAR 3.5), which we purchased as a positive control.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
HER2-HC-Me-SS-PBD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 44.00% (Day 24) | High HER2 expression (HER2+++) | ||
| Method Description |
All animals were euthanized before tumors reached 3000 mm3 or showed signs of impending ulceration. Mice were dosed IV via the tail vein with ADC conjugates A1-A4. A1 and A2 were dosed at 4 mg/kg.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
Anti-CLL-1-ds-PBD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 47.85% (Day 11) | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (0.25 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 57.73% (Day 14) | Moderate CLL-1 expression (CLL-1++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (0.75 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | THP1 CDX model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.19% (Day 14) | Moderate CLL-1 expression (CLL-1++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (1.5 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | THP1 CDX model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.28% (Day 11) | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (0.5 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.38% (Day 7) | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (0.25 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.48% (Day 14) | Moderate CLL-1 expression (CLL-1++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (3 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | THP1 CDX model | ||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.88% (Day 7) | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (0.5 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.23% (Day 7) | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (1 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.90% (Day 11) | Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Three human AML cell lines, were used to establish subcutaneous xenograft models for evaluation of anti-CLL-1-ds-PBD efficacy. When mean tumor size reached the desired volume (200 mm3), animals were randomized into groups of n = 8, each with similar mean tumor size. Four hours later, animals in each group were given a single intravenous dose of vehicle or ADC (1 mg/kg) through the tail vein.
Click to Show/Hide
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.00 ng/mL±2.00 ng/mL
|
Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Tumor cells were then blocked with excess amount of mouse IgG2a anti-ragweed antibody and treated with ADCs for 6 days at 37°C before cell viability was measured.
|
||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 ng/mL±2.00 ng/mL
|
Positive CLL-1 expression (CLL-1+++/++) | ||
| Method Description |
Tumor cells were then blocked with excess amount of mouse IgG2a anti-ragweed antibody and treated with ADCs for 6 days at 37°C before cell viability was measured.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [11] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
81.00 ng/mL±9.00 ng/mL
|
Moderate CLL-1 expression (CLL-1++) | ||
| Method Description |
Tumor cells were then blocked with excess amount of mouse IgG2a anti-ragweed antibody and treated with ADCs for 6 days at 37°C before cell viability was measured.
|
||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
HER2-LC-H-SS-PBD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.00% (Day 24) | High HER2 expression (HER2+++) | ||
| Method Description |
All animals were euthanized before tumors reached 3000 mm3 or showed signs of impending ulceration. Mice were dosed IV via the tail vein with ADC conjugates A1-A4. A1 and A2 were dosed at 4 mg/kg.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
HER2-HC-H-SS-PBD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.00% (Day 24) | High HER2 expression (HER2+++) | ||
| Method Description |
All animals were euthanized before tumors reached 3000 mm3 or showed signs of impending ulceration. Mice were dosed IV via the tail vein with ADC conjugates A1-A4. A1 and A2 were dosed at 4 mg/kg.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
HER2-LC-Me-SS-PBD [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [10] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 24) | High HER2 expression (HER2+++) | ||
| Method Description |
All animals were euthanized before tumors reached 3000 mm3 or showed signs of impending ulceration. Mice were dosed IV via the tail vein with ADC conjugates A1-A4. A1 and A2 were dosed at 4 mg/kg.
|
||||
| In Vivo Model | MMTV-HER2 Fo5 CDX model (Trastuzumab resistant) | ||||
| In Vitro Model | Breast cancer | MMTV-HER2 cells | Mus musculus | ||
WO2013055987A1 Tmab-110 [Investigative]
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 5.82% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 1 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 31.56% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 3 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 47.90% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 6 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
102.78 ug/mL
|
High HER2 expression (HER2 +++) | ||
| Method Description |
Certain cells are seeded at 1000-2000/well or 2000-3000/well in a 96-well plate, 50 uL/well. After one or two days, ADCs are added with "no ADC" control wells receiving medium alone. Conditions are in duplicate or triplicate After 3-5 days, 100 uL/well Cell TiterGlo ll isadded.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ug/mL | Negative HER2 expression (HER2 -) | ||
| Method Description |
Certain cells are seeded at 1000-2000/well or 2000-3000/well in a 96-well plate, 50 uL/well. After one or two days, ADCs are added with "no ADC" control wells receiving medium alone. Conditions are in duplicate or triplicate After 3-5 days, 100 uL/well Cell TiterGlo ll isadded.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
WO2013055987A1 Tmab-101 [Investigative]
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 78.27% (Day 11) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. When tumor sreached desired volumes, the tumor-bearing mice were randomized and given a single dose by IV 1 mg/kg injection of the ADC.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.68% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 1 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.51% (Day 11) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. When tumor sreached desired volumes, the tumor-bearing mice were randomized and given a single dose by IV 3 mg/kg injection of the ADC.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.92% (Day 11) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. When tumor sreached desired volumes, the tumor-bearing mice were randomized and given a single dose by IV 10 mg/kg injection of the ADC.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
22.12 ug/mL
|
High HER2 expression (HER2 +++) | ||
| Method Description |
Certain cells are seeded at 1000-2000/well or 2000-3000/well in a 96-well plate, 50 uL/well. After one or two days, ADCs are added with "no ADC" control wells receiving medium alone. Conditions are in duplicate or triplicate After 3-5 days, 100 uL/well Cell TiterGlo ll isadded.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ug/mL | Negative HER2 expression (HER2 -) | ||
| Method Description |
Certain cells are seeded at 1000-2000/well or 2000-3000/well in a 96-well plate, 50 uL/well. After one or two days, ADCs are added with "no ADC" control wells receiving medium alone. Conditions are in duplicate or triplicate After 3-5 days, 100 uL/well Cell TiterGlo ll isadded.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
WO2013055987A1 xCD22-103 [Investigative]
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [12] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 81.10% (Day 11) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. When tumor sreached desired volumes, the tumor-bearing mice were randomized and given a single dose by IV 10 mg/kg injection of the ADC.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
References
