Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0NIARR
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
WO2013055987A1 Tmab-110
|
|||||
| Synonyms |
WO2013055987A1 Tmab-110
Click to Show/Hide
|
|||||
| Organization |
ADC Therapeutics SA; Genentech, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
1.7
|
|||||
| Structure |
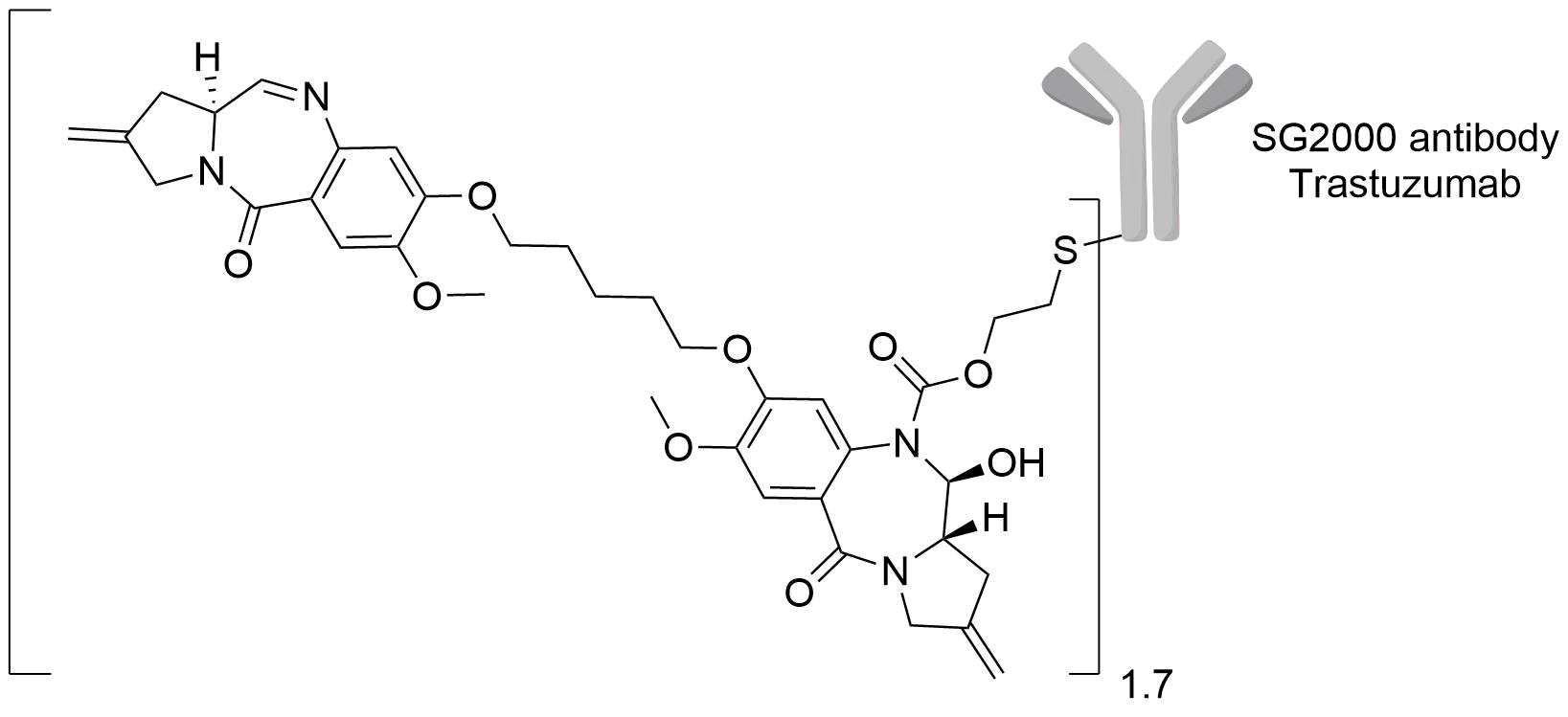
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
SG2000
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
WO2013055987A1_Tmab-110 linker
|
|||||
General Information of The Activity Data Related to This ADC
Obtained from the Model Organism Data
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 5.82% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 1 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 31.56% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 3 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 47.90% (Day 14) | High HER2 expression (HER2 +++) | ||
| Method Description |
Before being used for an in vivo efficacy study, the MMTV-HER2 Fo5 transgenic mammary tumor was surgically transplanted into the mammary fat pad of nu/nu mice in fragments that measured approximately 2x2 mm. MMTV-HER2 Fo5 mammary allograft tumors inoculated into CRL nu/nu mice aftersingle,then iv 6 mg/kg ADC dosing on day 0.
|
||||
| In Vivo Model | Breast cancer model MMTV-HER2 Fo5 | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 102.78 ug/mL | High HER2 expression (HER2 +++) | ||
| Method Description |
Certain cells are seeded at 1000-2000/well or 2000-3000/well in a 96-well plate, 50 uL/well. After one or two days, ADCs are added with "no ADC" control wells receiving medium alone. Conditions are in duplicate or triplicate After 3-5 days, 100 uL/well Cell TiterGlo ll isadded.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 ug/mL | Negative HER2 expression (HER2 -) | ||
| Method Description |
Certain cells are seeded at 1000-2000/well or 2000-3000/well in a 96-well plate, 50 uL/well. After one or two days, ADCs are added with "no ADC" control wells receiving medium alone. Conditions are in duplicate or triplicate After 3-5 days, 100 uL/well Cell TiterGlo ll isadded.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
