Payload Information
General Information of This Payload
| Payload ID | PAY0SIHVM |
|||||
|---|---|---|---|---|---|---|
| Name | Auristatin F hydroxypropylamide (AF-HPA) |
|||||
| Synonyms |
Auristatin f-hpa; Auristatin F hydroxypropylamide; 7A55BK9DV3; XMT-1267; 1415659-09-4; UNII-7A55BK9DV3; SCHEMBL14315837; L-PHENYLALANINAMIDE, N,N-DIMETHYL-L-VALYL-L-VALYL-(3R,4S,5S)-3-METHOXY-5-METHYL-4-(METHYLAMINO)HEPTANOYL-(.ALPHA.R,.BETA.R,2S)-.BETA.-METHOXY-.ALPHA.-METHYL-2-PYRROLIDINEPROPANOYL-N-(3-HYDROXYPROPYL)-; L-Phenylalaninamide, N,N-dimethyl-L-valyl-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(alphaR,betaR,2S)-beta-methoxy-alpha-methyl-2-pyrrolidinepropanoyl-N-(3-hydroxypropyl)-; N,N-DIMETHYL-L-VALYL-L-VALYL-(3R,4S,5S)-3-METHOXY-5-METHYL-4-(METHYLAMINO)HEPTANOYL-(.ALPHA.R,.BETA.R,2S)-.BETA.-METHOXY-.ALPHA.-METHYL-2-PYRROLIDINEPROPANOYL-N-(3-HYDROXYPROPYL)-L-PHENYLALANINAMIDE; N,N-Dimethyl-L-valyl-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(alphaR,betaR,2S)-beta-methoxy-alpha-methyl-2-pyrrolidinepropanoyl-N-(3-hydroxypropyl)-L-phenylalaninamide
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
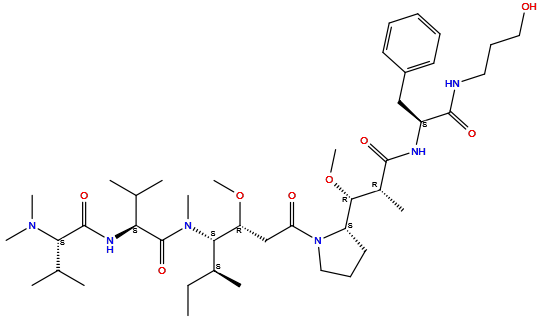
|
|||||
| Formula | C43H74N6O8 |
|||||
| Isosmiles | [H]OC([H])([H])C([H])([H])C([H])([H])N([H])C(=O)[C@@]([H])(N([H])C(=O)[C@]([H])(C([H])([H])[H])[C@@]([H])(OC([H])([H])[H])[C@@]1([H])N(C(=O)C([H])([H])[C@@]([H])(OC([H])([H])[H])[C@@]([H])(N(C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C1([H])[H])C([H])([H])c1c([H])c([H])c([H])c([H])c1[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C43H74N6O8/c1-13-29(6)38(48(10)43(55)36(27(2)3)46-42(54)37(28(4)5)47(8)9)34(56-11)26-35(51)49-23-17-21-33(49)39(57-12)30(7)40(52)45-32(41(53)44-22-18-24-50)25-31-19-15-14-16-20-31/h14-16,19-20,27-30,32-34,36-39,50H,13,17-18,21-26H2,1-12H3,(H,44,53)(H,45,52)(H,46,54)/t29-,30+,32-,33-,34+,36-,37-,38-,39+/m0/s1
|
|||||
| InChIKey |
XHXOHGJMQNOIIO-LMPBRMKVSA-N
|
|||||
| IUPAC Name |
(2S)-2-[[(2S)-2-(dimethylamino)-3-methylbutanoyl]amino]-N-[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(2S)-1-(3-hydroxypropylamino)-1-oxo-3-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-N,3-dimethylbutanamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
803.099 |
Polar area |
169.85 |
||
Complexity |
802.5568133 |
xlogp Value |
2.8598 |
|||
Heavy Count |
57 |
Rot Bonds |
37 |
|||
Hbond acc |
9 |
Hbond Donor |
4 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Upifitamab rilsodotin [Phase 3]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
34.00% (all)
35.00% (SLC34A2 high) 29.00% (SLC34A2 low) |
High SLC34A2 expression (SLC34A2+++; 66,000 SLC34A2 antigens/cell) | ||
| Patients Enrolled |
Ovarian cancer patients with 1-3 prior lines in platinum-resistant; 4 prior lines patients regardless of platinum status.
|
||||
| Administration Dosage |
36 or 43 mg/m 2 IV once every 4 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03319628 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Open-label, dose escalation to reach mtd. the mtd will be confirmed in parallel cohorts: patients with platinum-resistant ovarian cancer; patients with non-squamous nsclc, adenocarcinoma subtype.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05329545 | Phase Status | Phase 3 | ||
| Clinical Description |
A phase 3, randomized, double-blind, placebo-controlled, multicenter study of upifitamab rilsodotin (XMT-1536) as post-platinum maintenance therapy for participants with recurrent, platinum-sensitive, ovarian cancer (up-next).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Patients Enrolled |
Patients with a histological diagnosis of metastatic or recurrent high-grade serous ovarian cancer, including fallopian tube, or primary peritoneal cancer and have received 1-3 prior lines of therapy.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04907968 | Phase Status | Phase 1 | ||
| Clinical Description |
Upifitamab rilsodotin (XMT-1536) an open-label, multicenter, dose escalation and expansion study of upifitamab rilsodotin in combination with carboplatin in participants with high grade serous ovarian cancer (UP GRADE-A).
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 58.60% (Day 35) | Moderate SLC34A2 expression (SLC34A2++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0178) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.60% (Day 28) | Moderate SLC34A2 expression (SLC34A2++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0178) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.40% (Day 17) | Moderate SLC34A2 expression (SLC34A2++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: CTG-0860) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.10% (Day 28) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0852) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.40% (Day 35) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0852) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.70% (Day 62) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0852) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.00% (Day 28) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian adenocarcinoma CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.52 nM
|
|||
| Method Description |
OVCAR3 cells were grown in RPMI1640 media supplemented with 20% FBS and 1% penicillin/streptomycin,seeded at a density of 5, 000 cells per well in 100 L of growth media in a 96-well,white flat-bottom plate. Following overnight incubation,the media was replaced with 100 L of fresh media containing the test compounds at a 3-fold titration up to 33 nmol/L. The treated cells were incubated for 96 hours at 37°C in the presence of 5% CO2. In the OVCAR3 cell line,XMT-1536 was cytotoxic in a 96-hour cellular cytotoxicity assay.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
ASN-004 [Phase 1]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 28.20% (Day 21) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 0.3 mg/kg single dose.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 54.30% (Day 60) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was further evaluated in a tumor xenograft model derived from the H1975 human lung carcinoma cell line [5T4+; 15, 800 binding sites per cell]. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 0.3 mg/kg Q4D 4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung cancer | Lung cancer cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 55.50% (Day 12) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 1 mg/kg Q4D 4.
|
||||
| In Vivo Model | Cervical advanced-stage cancer CDX model | ||||
| In Vitro Model | Cervical advanced-stage cancer | Cervical advanced-stage cancer cells | Homo sapiens | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.20% (Day 21) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 1 mg/kg single dose.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.30% (Day 21) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 3 mg/kg single dose.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Negative 5T4 expression (5T4-) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. A tumor xenograft model derived from the NCI-N87 human gastric tumor cell line [5T4-; 4, 400 binding sites per cell] and high expression of HER2. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 3 mg/kg single dose.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric cancer | Gastric cancer cells | Homo sapiens | ||
| Experiment 7 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Negative 5T4 expression (5T4-) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. A tumor xenograft model derived from the NCI-N87 human gastric tumor cell line [5T4-; 4, 400 binding sites per cell] and high expression of HER2. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 3 mg/kg Q4D 3.
Click to Show/Hide
|
||||
| In Vivo Model | Gastric cancer CDX model | ||||
| In Vitro Model | Gastric cancer | Gastric cancer cells | Homo sapiens | ||
| Experiment 8 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was further evaluated in a tumor xenograft model derived from the H1975 human lung carcinoma cell line [5T4+; 15, 800 binding sites per cell]. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 1 mg/kg Q4D 4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung cancer | Lung cancer cells | Homo sapiens | ||
| Experiment 9 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 60) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was further evaluated in a tumor xenograft model derived from the H1975 human lung carcinoma cell line [5T4+; 15, 800 binding sites per cell]. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 3 mg/kg Q4D 4.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer CDX model | ||||
| In Vitro Model | Lung cancer | Lung cancer cells | Homo sapiens | ||
| Experiment 10 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 1 mg/kg Q4D 4.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 3 mg/kg Q4D 4.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 6mg/kg Q4D 4.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 40) | Low 5T4 expression (5T4+) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ASN004 was studied in a tumor xenograft model derived from the A431 (human cervical epidermoid,5T4+; 88, 000 binding sites per cell) tumor cell line. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 10 mg/kg single dose.
Click to Show/Hide
|
||||
| In Vivo Model | Cervical cancer CDX model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 70) | High 5T4 expression (5T4+++) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 1 mg/kg Q4D 4.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells (5T4 overexpression) | CVCL_0062 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 70) | High 5T4 expression (5T4+++) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 3 mg/kg Q4D 4.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells (5T4 overexpression) | CVCL_0062 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 70) | High 5T4 expression (5T4+++) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. Subcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 6mg/kg Q4D 4.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells (5T4 overexpression) | CVCL_0062 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 70) | High 5T4 expression (5T4+++) | ||
| Method Description |
ASN004 was evaluated for efficacy in human tumor mouse xenograft models,derived from four different human tumor cell types,having a wide range of 5T4 expression levels. ubcutaneous tumor xenografts were developed in nude mice with established mean tumor volumes of 150 mm3. The dose of ASN004 was 10 mg/kg single dose.
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells (5T4 overexpression) | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
High 5T4 expression (5T4+++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder squamous cell carcinoma | SCaBER cells | CVCL_3599 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.12 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | 5637 cells | CVCL_0126 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.12 nM
|
High 5T4 expression (5T4+++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Amelanotic melanoma | A-375 cells | CVCL_0132 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.17 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.18 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Endocervical adenocarcinoma | HeLa cells | CVCL_0030 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.23 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.26 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.31 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | SW780 cells | CVCL_1728 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.34 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Glioblastoma | U-87MG cells | CVCL_0022 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.42 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Prostate carcinoma | PC-3 cells | CVCL_0035 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.42 nM
|
High 5T4 expression (5T4+++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells (5T4 overexpression) | CVCL_0062 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.44 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Gestational choriocarcinoma | JEG-3 cells | CVCL_0363 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.56 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.57 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Colon cancer | HT29 cells | CVCL_A8EZ | ||
| Experiment 19 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.62 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.66 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | TCCSUP cells | CVCL_1738 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.76 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Recurrent bladder carcinoma | HT-1197 cells | CVCL_1291 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.78 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | RT-4 cells | CVCL_0036 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.93 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | T24 cells | CVCL_0554 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.95 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.03 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Breast ductal carcinoma | HCC1937 cells | CVCL_0290 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.05 nM
|
Low 5T4 expression (5T4+) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Lung large cell carcinoma | NCI-H1299 cells | CVCL_0060 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.06 nM
|
High 5T4 expression (5T4+++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | HT-1376 cells | CVCL_1292 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.34 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Lung small cell carcinoma | DMS 114 cells | CVCL_1174 | ||
| Experiment 29 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.53 nM
|
Negative 5T4 expression (5T4-) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | UM-UC-3 cells | CVCL_1783 | ||
| Experiment 30 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.28 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Bladder carcinoma | J82 cells | CVCL_0359 | ||
| Experiment 31 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.01 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Hepatoblastoma | Hep-G2 cells | CVCL_0027 | ||
| Experiment 32 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.03 nM
|
Moderate 5T4 expression (5T4++) | ||
| Method Description |
The cytotoxic effect of ASN004 was assessed in cell viability assays for a diverse panel of human solid tumor cell lines representing bladder,breast,cervical,colon,gastric,glioblastoma,liver,lung,melanoma,placenta,and prostate cancers.
|
||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
XMT-1592 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04396340 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1b, first-in-human, dose escalation and expansion study of XMT-1592 in patients with solid tumors likely to express NAPI2B.
|
||||
XMT-1522 [Phase 1 (Terminated)]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 49) | Positive HER2 expression (HER2+++/++) | ||
| Method Description |
XMT-1522 (3 mg/kg, every seven days x3) induces efficient tumor cell killing in cell line-derived models of JIMT-1 cells with HER2 expression with high expression.
|
||||
| In Vivo Model | JIMT-1 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
References
