Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0JNXDB
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
XMT-1592
|
|||||
| Synonyms |
XMT 1592;XMT1592; XMT-1592
Click to Show/Hide
|
|||||
| Organization |
Mersana Therapeutics, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
6
|
|||||
| Structure |
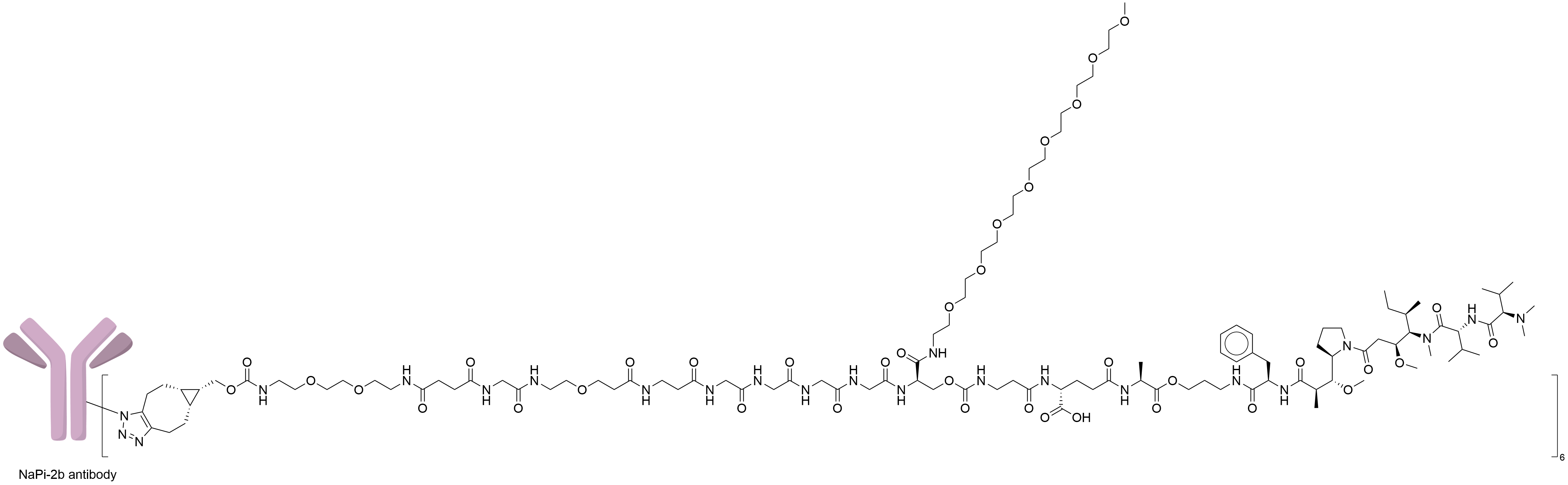
|
|||||
| Antibody Name |
Undisclosed
|
|||||
| Antigen Name |
Sodium-dependent phosphate transport protein 2B (SLC34A2)
|
Antigen Info | ||||
| Payload Name |
Auristatin F hydroxypropylamide (AF-HPA)
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Cys-13 ADC linker
|
Linker Info | ||||
| Conjugate Type |
Enzymatic Catalysis
|
|||||
| Puchem SID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04396340 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1b, first-in-human, dose escalation and expansion study of XMT-1592 in patients with solid tumors likely to express NAPI2B. | ||||
References
