Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ZROBT
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
CD19-ATAC
|
|||||
| Synonyms |
CD19ATAC; CD19 ATAC
Click to Show/Hide
|
|||||
| Organization |
Heidelberg Pharma AG
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
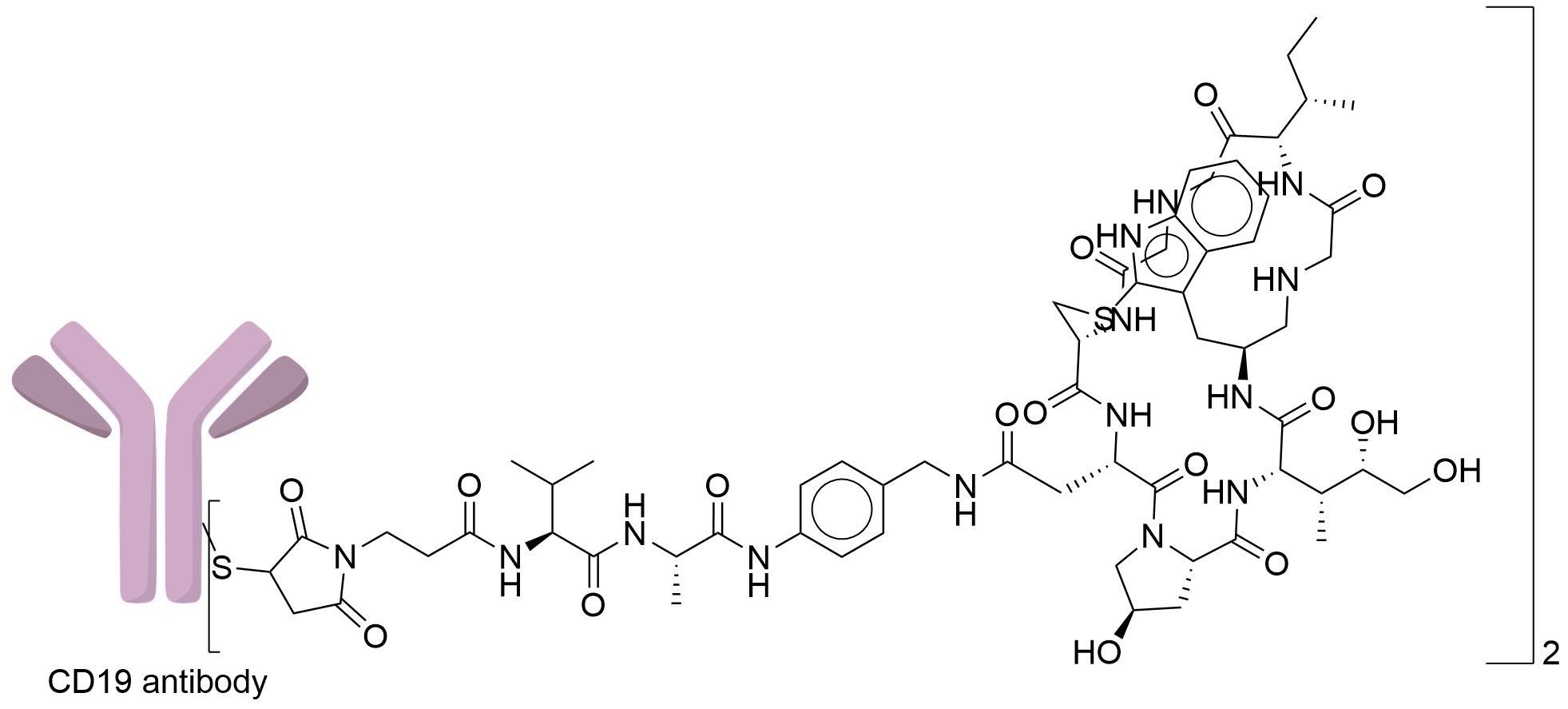
|
|||||
| Antibody Name |
Undisclosed
|
|||||
| Antigen Name |
B-lymphocyte antigen CD19 (CD19)
|
Antigen Info | ||||
| Payload Name |
HDP 30.2115
|
Payload Info | ||||
| Therapeutic Target |
DNA-directed RNA polymerase II subunit RPB2 (POLR2B); DNA-directed RNA polymerase III subunit RPC7 (POLR3G)
|
Target Info | ||||
| Linker Name |
Mal-Val-Ala-PAB
|
Linker Info | ||||
| Conjugate Type |
Site-specific conjugation through the engineered cysteine (THIOMAB).
|
|||||
