Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ZQBSG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MRG-001
|
|||||
| Synonyms |
MRG 001; MRG-001; MRG001
Click to Show/Hide
|
|||||
| Organization |
Shanghai Miracogen Inc.; Lepu Biopharma Co., Ltd.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
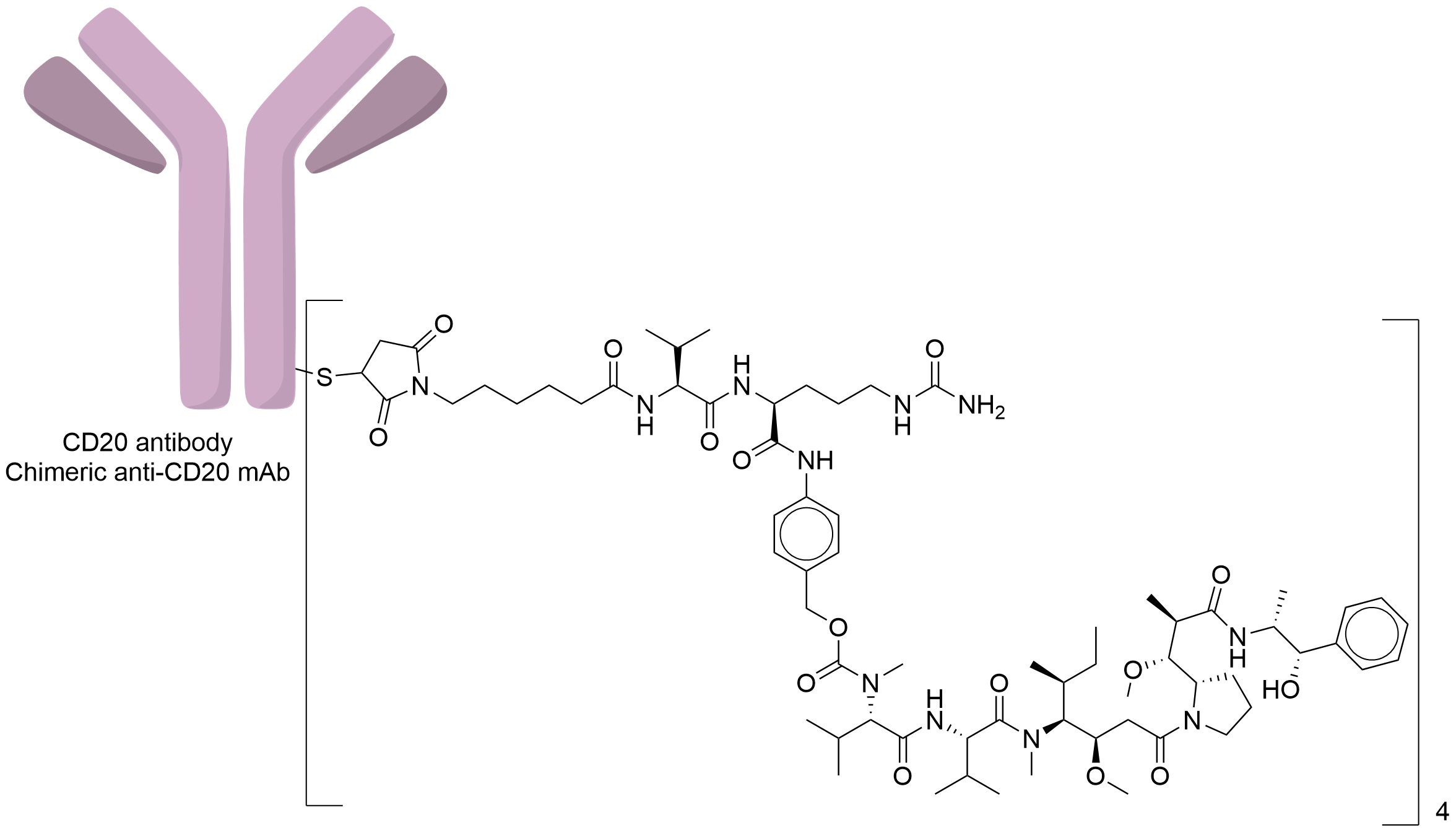
|
|||||
| Antibody Name |
Chimeric Anti-CD20 mAb
|
Antibody Info | ||||
| Antigen Name |
B-lymphocyte antigen CD20 (MS4A1)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
23.81% (among 21 pts who had at least one tumor assessment)
33.33% (in the 1.8 mg/kg cohort, DLBCL) 66.7% ( in the 2.5 mg/kg cohort, FL) |
|||
| Patients Enrolled |
CD20-positive relapsed or refractory (R/R) B-cell non Hodgkin lymphoma (NHL).
|
||||
| Administration Dosage |
Six dose levels ranging from 0.15 to 2.50 mg/kg, intravenously once every 3 weeks (Q3W) for a maximum of 6 treatment cycles.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05155839 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, multicenter, first-in-human, phase 1 dose-escalation and expansion clinical study to assess the safety, tolerability, pharmacokinetics and preliminary efficacy of MRG001 in patients with CD20-positive relapsed or refractory B-cell non-Hodgkin lymphoma (NHL). | ||||
| Primary Endpoint |
Rp2D=1.80 mg/kg.
|
||||
| Other Endpoint |
Among 21 pts who had at least one tumor assessment, ORR=23.81% , PR rate=19.05% (N=4),CR rate=4.76% (N=1).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05844527 | Clinical Status | Phase 2 | ||
| Clinical Description | Safety and efficacy of MRG-001 in wound healing in pre-abdominoplasty surgical excisions and scar appearance in subjects undergoing abdominoplasty. | ||||
References
