Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ZIJNR
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
hLL1-DOX
|
|||||
| Synonyms |
CD-74-DOX; IMMU-110; Milatuzumab doxorubicin; Milatuzumab-DOX; Milatuzumab-doxorubicin conjugate; hCD74-DOX; hLL1-DOX; hLL1-DOX, IMMU-115; hLL1-doxorubicin
Click to Show/Hide
|
|||||
| Organization |
Immunomedics, Inc.; Gilead Sciences, Inc.
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
6 to 8
|
|||||
| Structure |
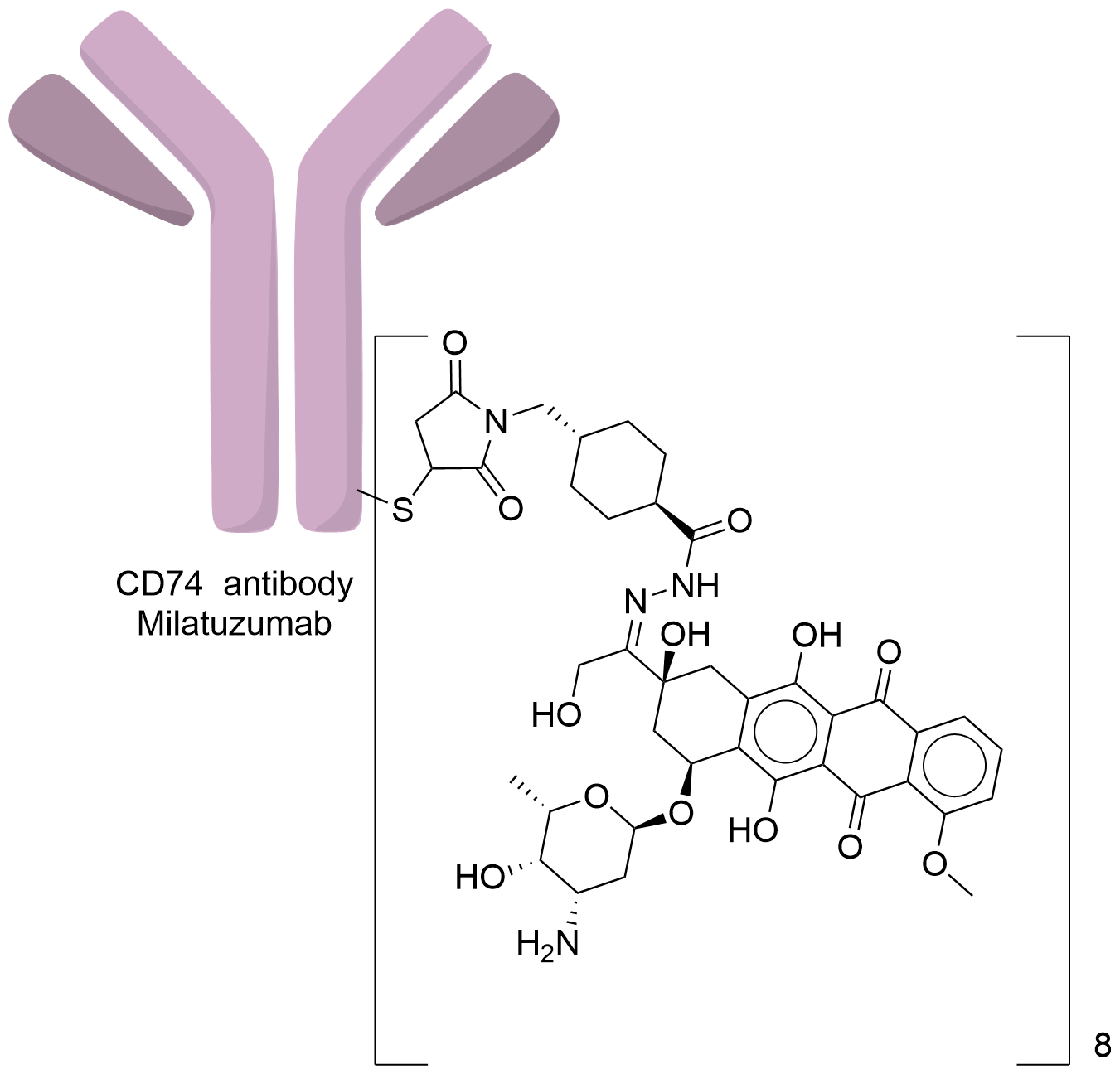
|
|||||
| Antibody Name |
Milatuzumab
|
Antibody Info | ||||
| Antigen Name |
HLA class II histocompatibility antigen gamma chain (CD74)
|
Antigen Info | ||||
| Payload Name |
Doxorubicin
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 2-alpha (TOP2A)
|
Target Info | ||||
| Linker Name |
Mcc-hydrazide
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
General Information of The Activity Data Related to This ADC
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.80 uM | Positive CD74 expression (CD74+++/++) | ||
| Method Description |
Briefly, cells were placed in 96-well plates (2 x105 per well) and subsequently incubated with serial dilutions of IMMU-110,naked hLL1,nonspecific negative control mAb-drug conjugate (hRS7-doxorubicin),or nonspecific mAb (hRS7) on ice for 1.5 hours. 4-hour IC50 values of IMMU-110,hRS7-doxorubicin,and free doxorubicin against the multiple myeloma cell line (MC/CAR) and other CD74-positive non-Hodgkin's lymphoma cell lines (Daudi and Raji).
Click to Show/Hide
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.90 uM | Positive CD74 expression (CD74+++/++) | ||
| Method Description |
Briefly, cells were placed in 96-well plates (2 x105 per well) and subsequently incubated with serial dilutions of IMMU-110,naked hLL1,nonspecific negative control mAb-drug conjugate (hRS7-doxorubicin),or nonspecific mAb (hRS7) on ice for 1.5 hours. 4-hour IC50 values of IMMU-110,hRS7-doxorubicin,and free doxorubicin against the multiple myeloma cell line (MC/CAR) and other CD74-positive non-Hodgkin's lymphoma cell lines (Daudi and Raji).
Click to Show/Hide
|
||||
| In Vitro Model | Normal | MC/CAR cells | CVCL_1397 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 1.50 uM | Positive CD74 expression (CD74+++/++) | ||
| Method Description |
Briefly, cells were placed in 96-well plates (2 x105 per well) and subsequently incubated with serial dilutions of IMMU-110,naked hLL1,nonspecific negative control mAb-drug conjugate (hRS7-doxorubicin),or nonspecific mAb (hRS7) on ice for 1.5 hours. 4-hour IC50 values of IMMU-110,hRS7-doxorubicin,and free doxorubicin against the multiple myeloma cell line (MC/CAR) and other CD74-positive non-Hodgkin's lymphoma cell lines (Daudi and Raji).
Click to Show/Hide
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
References
