Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0ZGAYH
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
LR004-Val-Cit-MMAE
|
|||||
| Synonyms |
LR004-Val-Cit-MMAE
Click to Show/Hide
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4.02
|
|||||
| Structure |
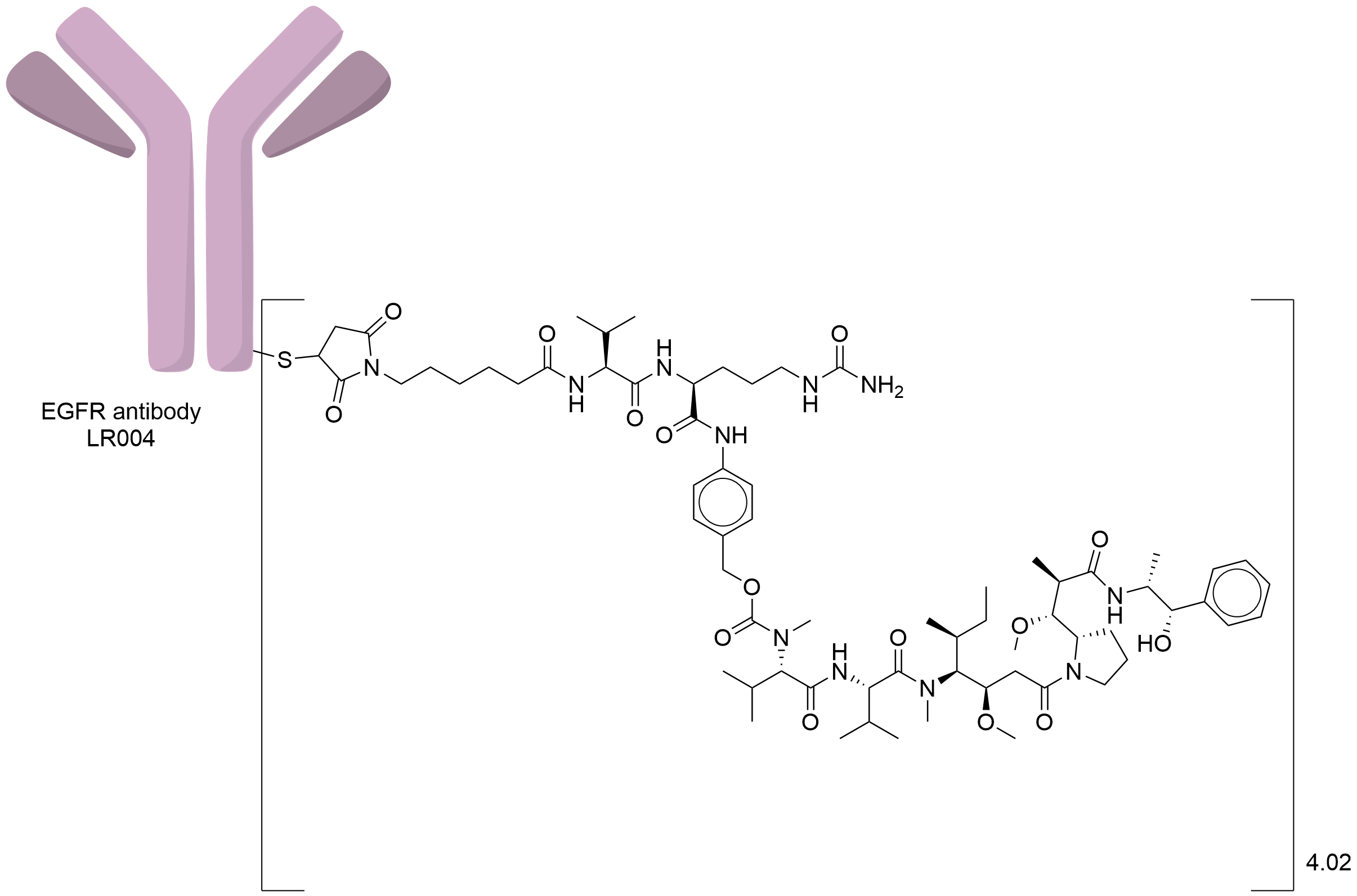
|
|||||
| Antibody Name |
LR004
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 52) | High EGFR expression (EGFR+++) | ||
| Method Description |
For the tumor xenograft model, 5.0x106 MDA-MB-468 cells or 3.0x106 MDA-MB-231 cells were injected subcutaneously into the right flank of each 6 week-old female BALB/c nude mice. when the tumor volumes reached approximately 100 mm3 (approximately 8 days), the 24 mice were randomly divided into 4 groups (6 mice per group): control group, LR004 antibody group, LR004-VC-MMAE group, and doxorubicin (positive control) group, which were treated with PBS or different drugs (LR004 antibody 10 mg/kg, LR004-VC-MMAE 10 mg/kg, doxorubicin 2 mg/kg) every 5 days for 4 times through i.v. injections.
Click to Show/Hide
|
||||
| In Vivo Model | Triple-negative breast cancer CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
