Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WOUMV
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
DLYE5953A
|
|||||
| Synonyms |
Anti-Ly6E; DLYE-5953A; DLYE59 53A, RG784 1; DLYE5953A; RG 7841; RG-7841
Click to Show/Hide
|
|||||
| Organization |
Genentech, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
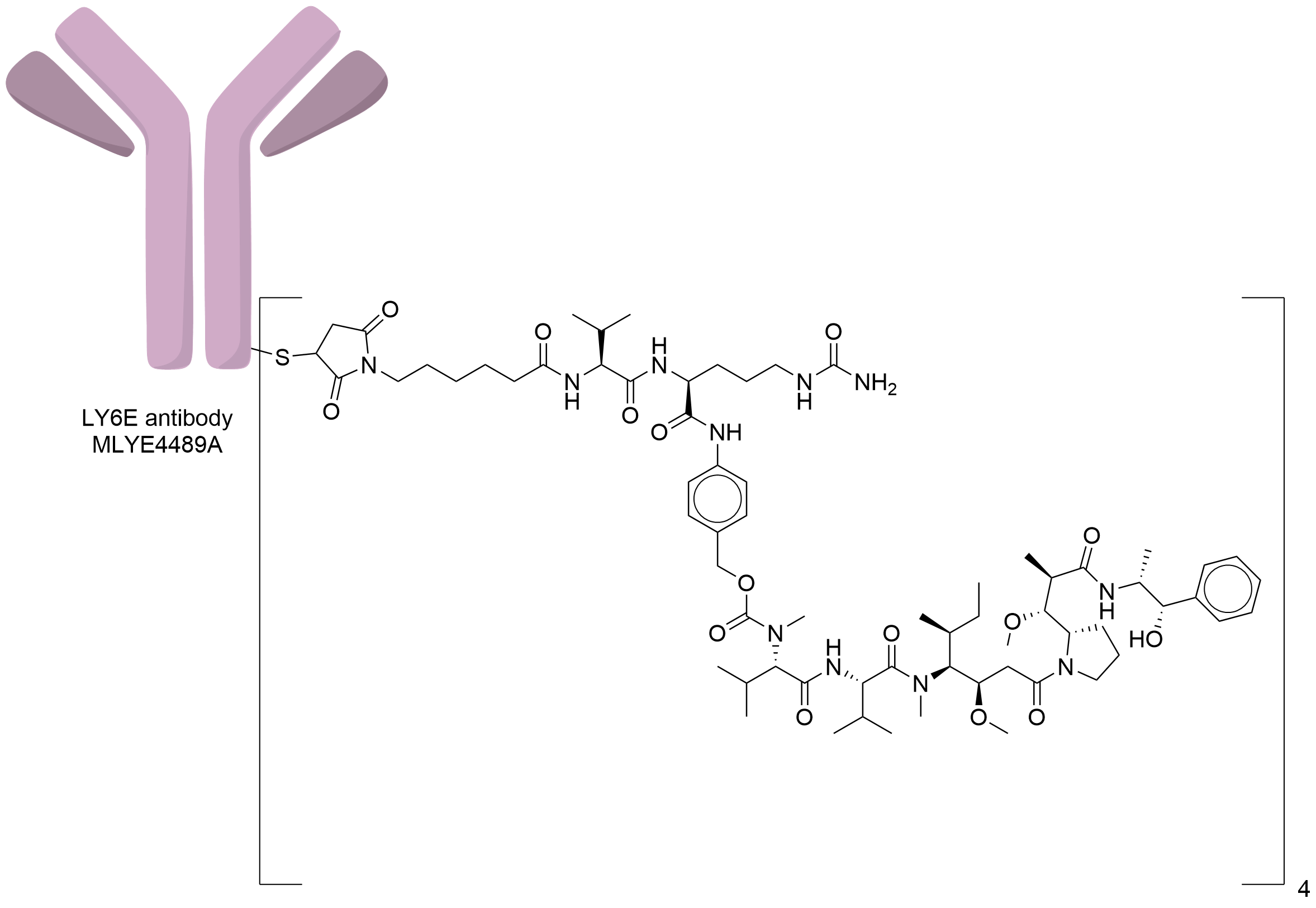
|
|||||
| Antibody Name |
MLYE4489A
|
Antibody Info | ||||
| Antigen Name |
Lymphocyte antigen 6E (LY6E)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
vedotin
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
17.76%
|
|||
| Patients Enrolled |
Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, histologically or cytologically documented advanced or metastatic breast, ovarian, pancreatic, NSCLC, head and neck squamous cell carcinoma, or gastric cancer in dose escalation, adequate hematologic and end organ function, and measurable disease per RECIST v1.1.
|
||||
| Administration Dosage |
Intravenously at doses of 0.20, 0.40, 0.80, 1.60, or 2.40 mg/kg once every 3 weeks (Q3W).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02092792 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label study evaluating the safety and tolerability of escalating doses of DLYE5953A in patients with refractory solid tumors. | ||||
| Primary Endpoint |
The confirmed overall objective response rate was 17.76%. All responders (8/68 patients) had PR,and all had received the RP2D dose of 2.40 mg/kg. This included three patients with MBC, and five patients in the NSCLC expansion cohort (5/25; 20%).
|
||||
| Other Endpoint |
The recommended phase II dose (RP2D) was 2.40 mg/kg Q3W. No dose-limiting toxicities were identified during dose escalation (0.20-2.40 mg/kg; n = 20).
|
||||
References
