Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WIBDT
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
HDP-101
|
|||||
| Synonyms |
HDP 101; HDP-101; HDP101
Click to Show/Hide
|
|||||
| Organization |
Heidelberg Pharma AG; Huadong Medicine Co., Ltd.; Hangzhou Zhongmeihuadong Pharmaceutical Co., Ltd.; Heidelberg Pharma Research GmbH
|
|||||
| Drug Status |
Phase 1/2
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
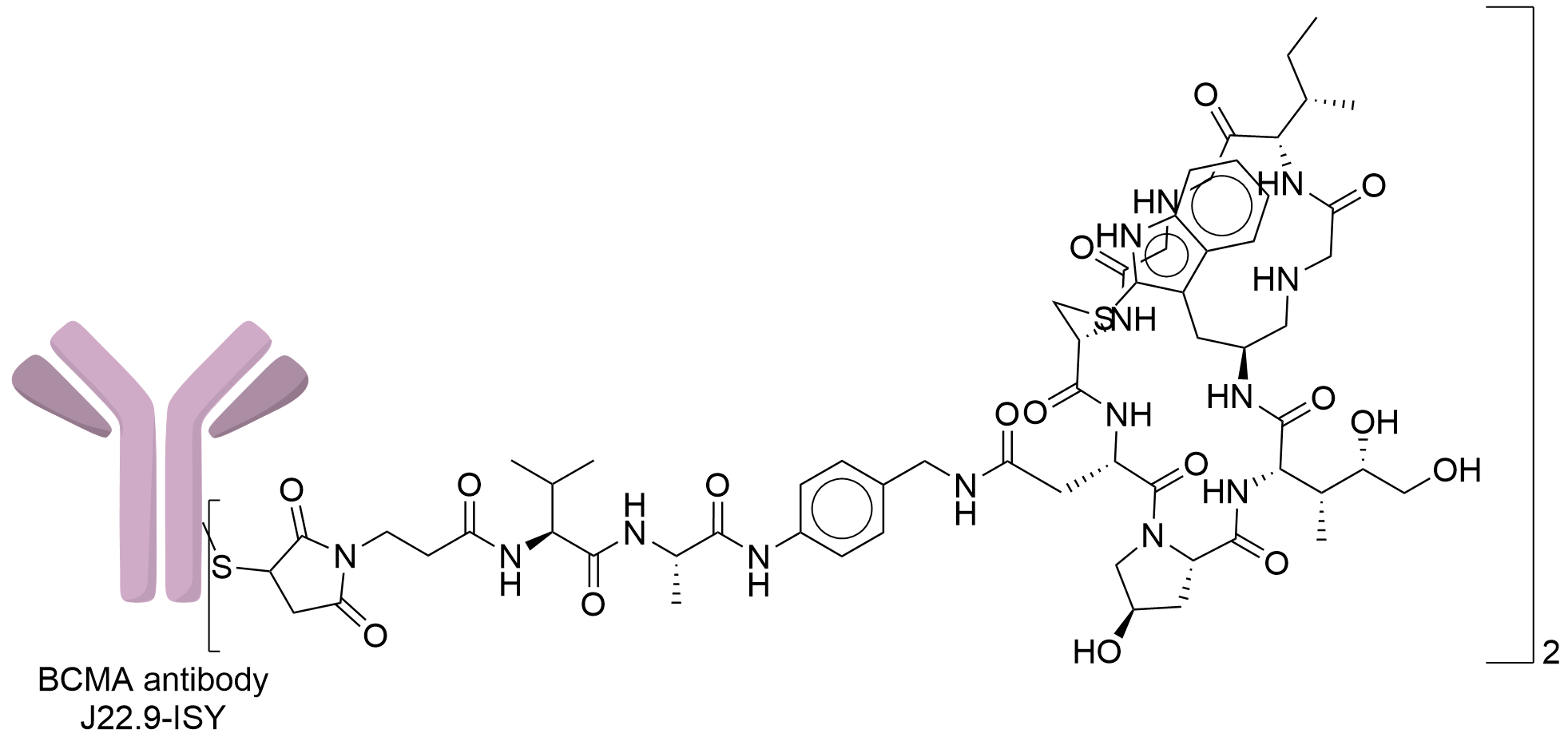
|
|||||
| Antibody Name |
J22.9-ISY
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor receptor superfamily member 17 (TNFRSF17)
|
Antigen Info | ||||
| Payload Name |
HDP 30.2115
|
Payload Info | ||||
| Therapeutic Target |
DNA-directed RNA polymerase II subunit RPB2 (POLR2B); DNA-directed RNA polymerase III subunit RPC7 (POLR3G)
|
Target Info | ||||
| Linker Name |
Mal-Val-Ala-PAB
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
tismanitin
|
|||||
General Information of The Activity Data Related to This ADC
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | U266B1 cells | CVCL_0566 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | NCI-H929 cells | CVCL_1600 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.16 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | MM1.S cells | CVCL_8792 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.18 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | INA-6 cells | CVCL_5209 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | LP-1 cells | CVCL_0012 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.34 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Multiple myeloma | SK-MM-1 cells | CVCL_A478 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
4.83 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | L-363 cells | CVCL_1357 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
34.20 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | OPM-2 cells | CVCL_1625 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
142.00 nM
|
|||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Plasma cell myeloma | RPMI-8226 cells | CVCL_0014 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1000.00 nM | |||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Normal | HS-5 cells | CVCL_3720 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1000.00 nM | |||
| Method Description |
The inhibitory activity of HDP-101 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 2 or 4 days.
|
||||
| In Vitro Model | Osteosarcoma | U2OS cells | CVCL_0042 | ||
References
