Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WCTHL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Pivekimab sunirine
|
|||||
| Synonyms |
IMGN 632; IMGN-632; IMGN632; Pivekimab sunirine
Click to Show/Hide
|
|||||
| Organization |
ImmunoGen, Inc.
|
|||||
| Drug Status |
Phase 1/2
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
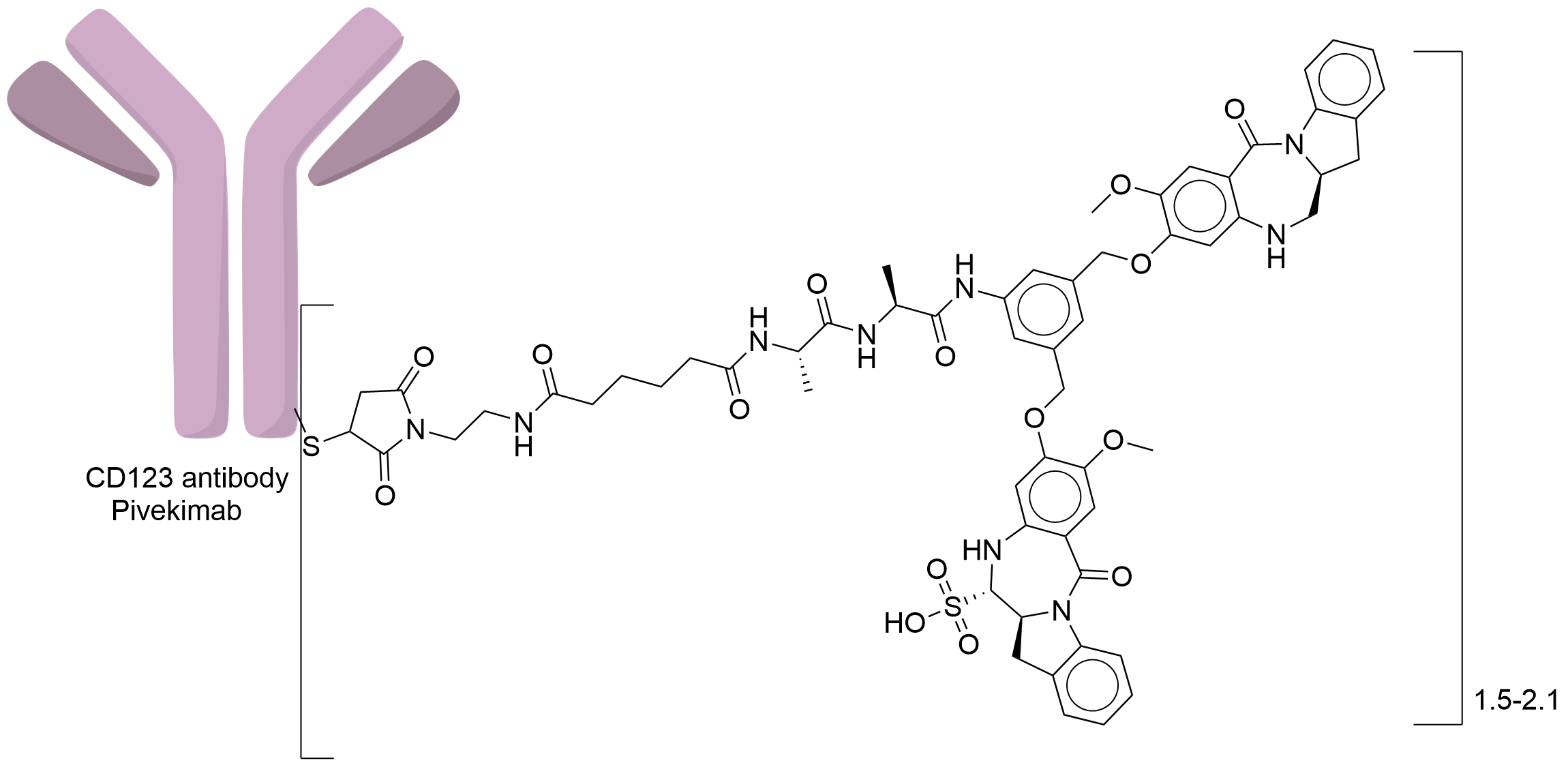
|
|||||
| Antibody Name |
Pivekimab
|
Antibody Info | ||||
| Antigen Name |
Interleukin-3 receptor subunit alpha (IL3RA)
|
Antigen Info | ||||
| Payload Name |
sulfonated DGN462
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mal-adipamide-Ala-Ala
|
Linker Info | ||||
| Conjugate Type |
Reactive Cysteines
|
|||||
| Combination Type |
sunirine
|
|||||
| Special Approval(s) |
Accelerated approval(FDA); Orphan drug(FDA); Orphan drug(EMA)
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
