Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WCBFK
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Cirmtuzumab vedotin
|
|||||
| Synonyms |
UC-961ADC3
Click to Show/Hide
|
|||||
| Organization |
University of California; Oncternal Therapeutics, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 6 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2.5
|
|||||
| Structure |
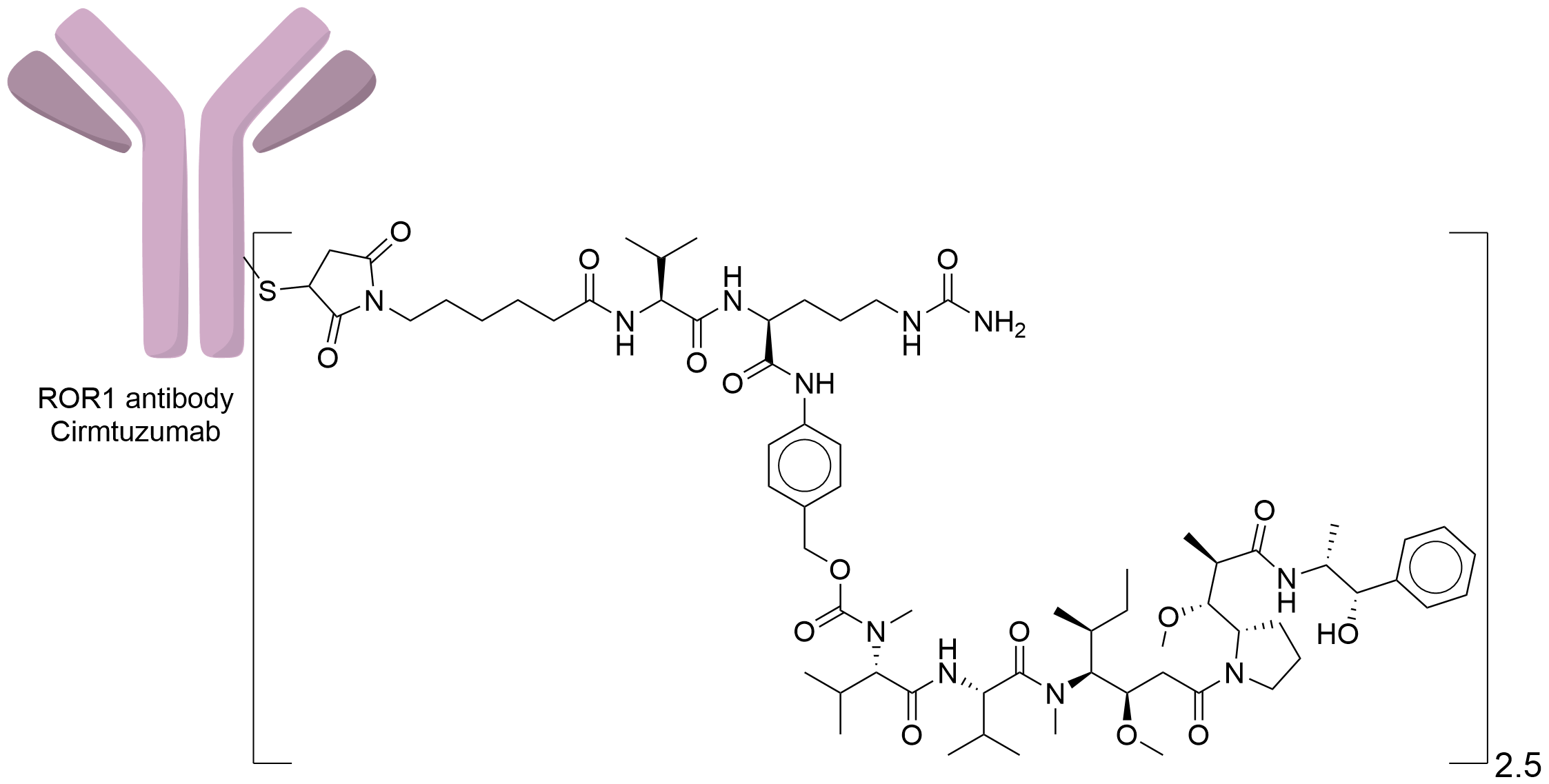
|
|||||
| Antibody Name |
Cirmtuzumab
|
Antibody Info | ||||
| Antigen Name |
Inactive tyrosine-protein kinase transmembrane receptor ROR1 (ROR1)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Lysine-linker
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
