Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0UQTJG
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Lifastuzumab vedotin
|
|||||
| Synonyms |
Anti-NaPi2b ADC; DNIB-0600A; DNIB0600A; LIFA; Lifastuzumab vedotin; NaPi2b-ADC; NaPi3b; RG-7599; RG7599, DNIB-0600A; RO-5541081
Click to Show/Hide
|
|||||
| Organization |
Genentech, Inc.; Roche Holding AG; Roche Holding AG
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3 to 4
|
|||||
| Structure |
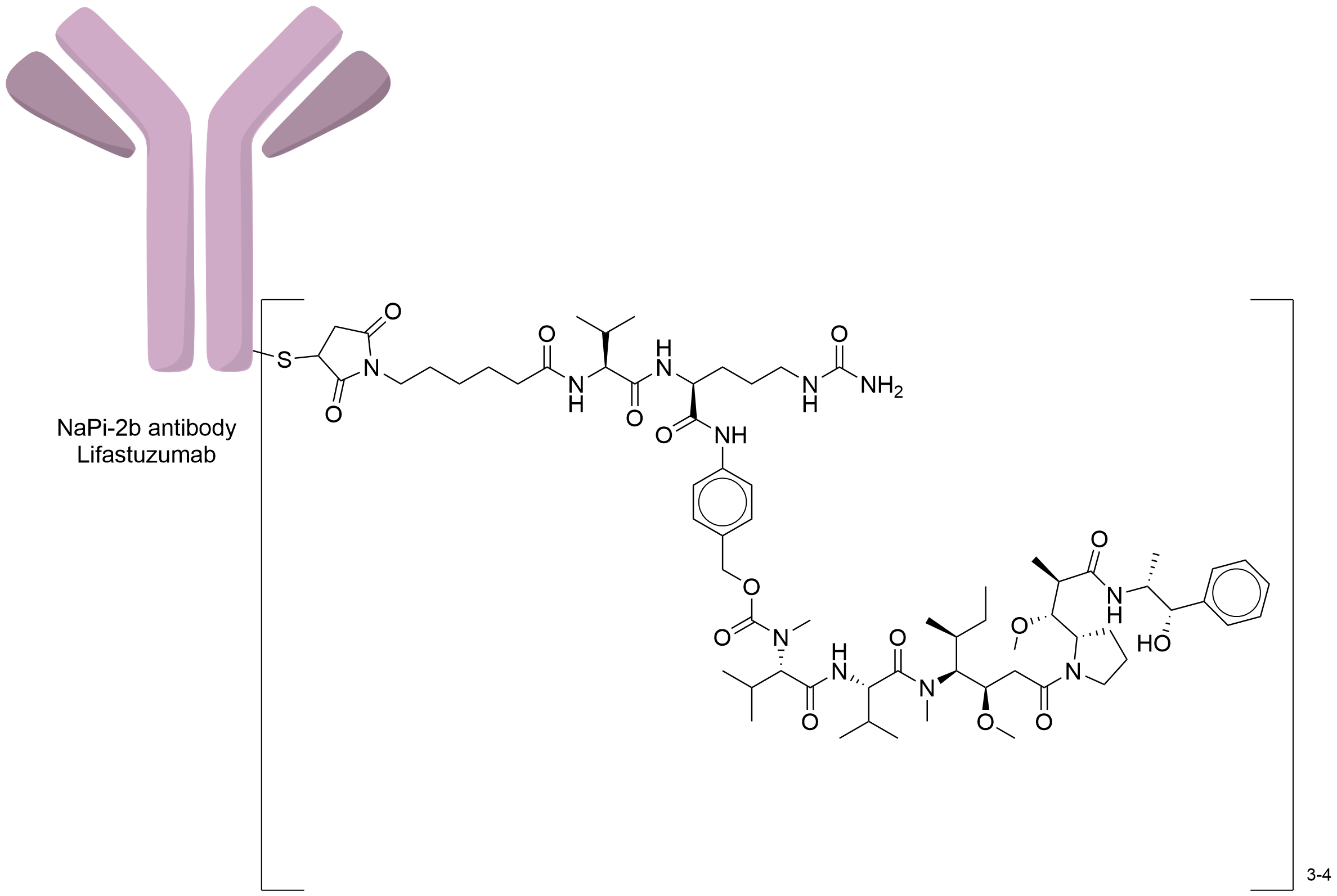
|
|||||
| Antibody Name |
Lifastuzumab
|
Antibody Info | ||||
| Antigen Name |
Sodium-dependent phosphate transport protein 2B (SLC34A2)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
vedotin
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Tumor Growth Inhibition value (TGI) |
≈ 41.2
|
%
|
OVCAR-3 cells
|
Ovarian serous adenocarcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
7.84% (patients with NSCLC, active doses 1.8 mg/kg)
45.83% (patients with PROC, active doses 1.8 mg/kg) 25.00% (1.8 mg/kg, in the NSCLC cohorts) 6.67% (2.4 mg/kg, in the NSCLC cohorts) |
|||
| Patients Enrolled |
Incurable, locally advanced, or metastatic disease (non-squamous NSCLC or non-mucinous PROC) that had progressed on or following prior chemotherapy and for which no standard therapy existed, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
|
||||
| Administration Dosage |
The starting dose for LIFA was 0.20 mg/kg administered by intravenous infusion (IV) every 3 weeks (Q3W), in this 3+3 dose-escalation design, followed by cohort expansion at the recommended phase II dose (RP2D).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01911598 | Clinical Status | Phase 1a | ||
| Clinical Description | A phase 1b open-label study of the safety and pharmacokinetics of MEHD7945A in combination with either cisplatin and 5-fu or paclitaxel and carboplatin in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. | ||||
| Primary Endpoint |
The MTD was not reached on this study and the maximum administered dose (MAD) was 2.80 mg/kg. Upon evaluation of the safety data of the 6 patients treated at the MAD, the dose of 2.40 mg/kg was established as the RP2D.
|
||||
| Other Endpoint |
At active doses 1.80 mg/kg, partial responses were observed in four of 51 (7.84%) patients with NSCLC and 11 of 24 (45.83%) patients. In the NSCLC cohorts, PRs were seen in 1 of 4 (25.00%) at 1.80 mg/kg and in 3 of 45 (6.67%) patients at 2.40 mg/kg, mDoR=161 days. In PROC, PRs were seen at 1.80 mg/kg in one of two (50.00%), at 2.40 mg/kg in seven of 18 (38.89%), and at 2.80 mg/kg in three of four (75.00%) patients; mDoR=342 days.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
34.00% (ITT population)
36.00% (NaPi2bx high patients) |
|||
| Patients Enrolled |
Advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer that had progressed or relapsed within 6months of the most recent treatment with a platinum-containing chemotherapy regimen and for whom PLD was considered an appropriate therapy.
|
||||
| Administration Dosage |
2.40 mg/kg, intravenously, every 3 weeks (Q3W).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01991210 | Clinical Status | Phase 2 | ||
| Clinical Description | A randomized, open-label, multicenter, phase 2 trial evaluating the safety and activity of DNIB0600A compared to pegylated liposomal doxorubicin administered intravenously to patients with platinum-resistant ovarian cancer. | ||||
| Primary Endpoint |
The stratified PFS hazard ratio was 0.78 (95% CI,0.46-1.31; p=0.34) with a median PFS of 5.30 vs. 3.10 months (LIFA vs. PLD arm,respectively) in the ITT population, and 0.71 (95% CI,0.40-1.26; p=0.24) with a median PFS of 5.30 vs. 3.40 months (LIFA vs. PLD arm,respectively) in NaPi2bxhigh patients. The objective response rate (ORR) was 34.00% (95% CI,22.00-49.00%,LIFA) vs. 15.00% (95% CI, 7.00-28.00%,PLD) in the ITT population (p=0.03), and 36.00% (95% CI, 22.00-52.00%,LIFA) vs.14.00% (95% CI,6.00-27.00%,PLD) in NaPi2bxhigh patients (p=0.02).
Click to Show/Hide
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
34.00% (all)
36.00% (SLC34A2 2+/3+) |
|||
| Patients Enrolled |
Platinum-resistant patients with histologically documented advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer.
|
||||
| Administration Dosage |
2.40 mg/kg, intravenously, every 3 weeks (Q3W).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01991210 | Clinical Status | Phase 2 | ||
| Clinical Description | A randomized, open-label, multicenter, phase 2 trial evaluating the safety and activity of DNIB0600A compared to pegylated liposomal doxorubicin administered intravenously to patients with platinum-resistant ovarian cancer. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
25.00% (1.8 mg/kg NSCLC)
7.00% (2.4 mg/kg NSCLC) 50.00% (1.8 mg/kg PROC) 39.00% (2.4 mg/kg PROC) 75.00% (2.8 mg/kg PROC) |
|||
| Patients Enrolled |
Patients with nonsmall cell lung cancer (NSCLC) and platinum-resistant ovarian cancer (PROC).
|
||||
| Administration Dosage |
Dose-escalation: 0.20-2.80 mg/kg once every 3 weeks, dose-expansion: 4 mg/kg once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01363947 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label study of the safety and pharmacokinetics of escalating doses of DNIB0600A in patients with non-small cell lung cancer and platinum-resistant ovarian cancer. | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Complete Remission (CR) |
58.54%
|
|||
| Patients Enrolled |
Polycystic ovary syndrome (PSOC) patients (epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer) with documented radiographic progression or relapse according to RECIST v1.1.
|
||||
| Administration Dosage |
The starting dose of LIFA was 1.20 mg/kg, once every 3 weeks (Q3W) on the first day of 21-day cycles, traditional 3 + 3 study design.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01995188 | Clinical Status | Phase 1b | ||
| Clinical Description | A phase 1b, open-label, dose-escalation study of the safety and pharmacology of DNIB0600A in combination with carboplatin (with or without bevacizumab) in patients with platinum-sensitive ovarian cancer or non-squamous non-small cell lung cancer. | ||||
| Primary Endpoint |
The median duration of progression-free survival was 10.71 months (95% CI: 8.54,13.86) with confirmed complete/partial responses in 24 (58.54%) patients.
|
||||
| Other Endpoint |
The maximum tolerated dose was not reached. The recommended phase 2 dose (RP2D) was LIFA 2.40 mg/kg + carboplatin AUC6 (cycles 16), with or without bevacizumab 15 mg/kg.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT01995188 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1b, open-label, dose-escalation study of the safety and pharmacology of DNIB0600A in combination with carboplatin (with or without bevacizumab) in patients with platinum-sensitive ovarian cancer or non-squamous non-small cell lung cancer. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 19.60% (Day 17) | Moderate SLC34A2 expression (SLC34A2++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Non-small cell lung cancer PDX model (PDX: CTG-0860) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 25.40% (Day 35) | Moderate SLC34A2 expression (SLC34A2++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0178) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 27.60% (Day 28) | Moderate SLC34A2 expression (SLC34A2++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0178) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 59.30% (Day 62) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0852) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.70% (Day 35) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0852) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 62.80% (Day 28) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg qwk x 3.
Click to Show/Hide
|
||||
| In Vivo Model | Lung cancer PDX model (PDX: CTG-0852) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 41.20% (Day 28) | High SLC34A2 expression (SLC34A2+++) | ||
| Method Description |
Animals were randomized into treatment groups (n = 10) when the tumor target volume reached 100-150 mm3. Test articles were administered intravenously via tail vein injection. Mice received a single dose of either saline vehicle; XMT-1535 at 3 mg/kg; XMT-1536 (DAR 12.4) at 3 mg/kg; IgG1-Dolaflexin (DAR 18.1) at 3 mg/kg,or lifastuzumab vedotin (DAR 4.1) at 3 mg/kg. Tumors were measured twice per week. XMT-1536 and lifastuzumab vedotin were administered at a single dose of 3 mg/kg.
Click to Show/Hide
|
||||
| In Vivo Model | Ovarian adenocarcinoma CDX model | ||||
| In Vitro Model | Ovarian serous adenocarcinoma | OVCAR-3 cells | CVCL_0465 | ||
References
