Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0TTTUA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
SHR-A2009
|
|||||
| Synonyms |
SHR A2009; SHR-A2009; SHRA2009
Click to Show/Hide
|
|||||
| Organization |
Suzhou Suncadia Biopharmaceuticals Co., Ltd.
|
|||||
| Drug Status |
Phase 3
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
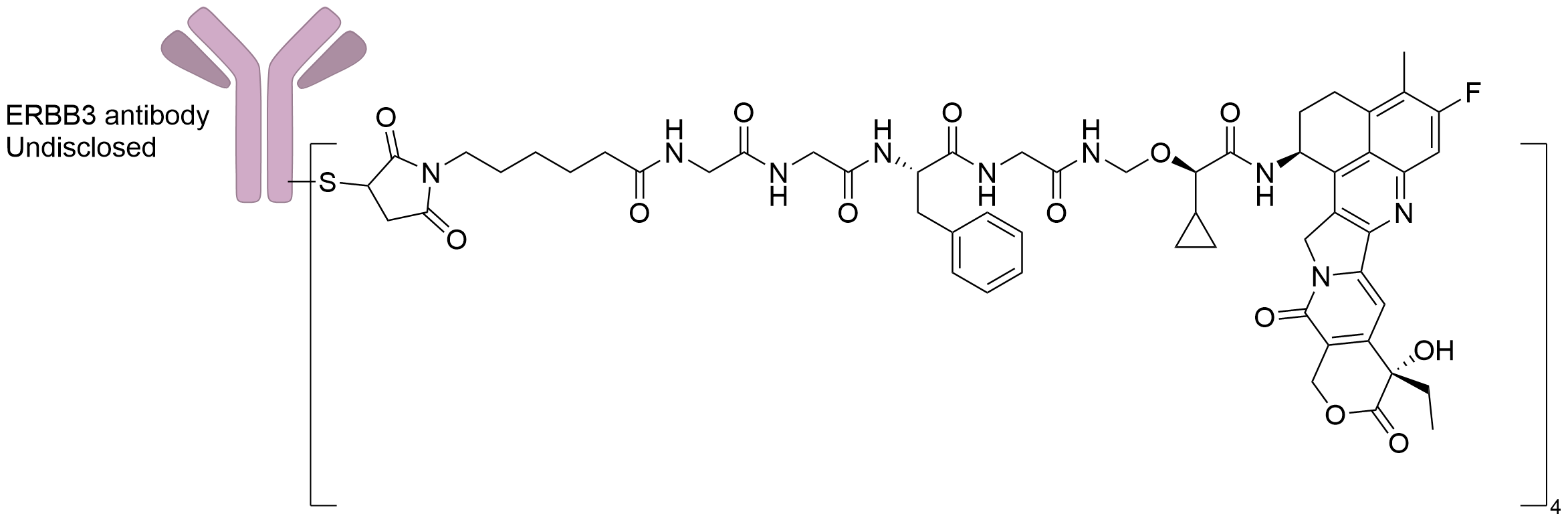
|
|||||
| Antibody Name |
Ruzaltatug
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-3 (HER3)
|
Antigen Info | ||||
| Payload Name |
9106-IM-2
|
Payload Info | ||||
| Therapeutic Target |
DNA topoisomerase 1 (TOP1)
|
Target Info | ||||
| Linker Name |
Mc-Gly-Gly-Phe-Gly
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
SHR169106
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05394818 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetics and efficacy of SHR-A2009 for injection in patients with advanced solid tumors. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05114759 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label, multicenter clinical study to evaluate the safety, tolerability, pharmacokinetics and efficacy of SHR-A2009 for injection in patients with advanced solid tumors. | ||||
References
