Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0TIZXC
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Trastuzumab botidotin
|
|||||
| Brand Name |
Sertaly
|
|||||
| Synonyms |
A 166; A-166; A166; KL-A166
Click to Show/Hide
|
|||||
| Organization |
Sichuan Kelun Pharmaceutical Co., Ltd.; Fudan University; KLUS Pharma, Inc.; Levena Biopharma US, Inc.
|
|||||
| Drug Status |
Approved (FDA): Oct, 2025
|
|||||
| Indication |
In total 8 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
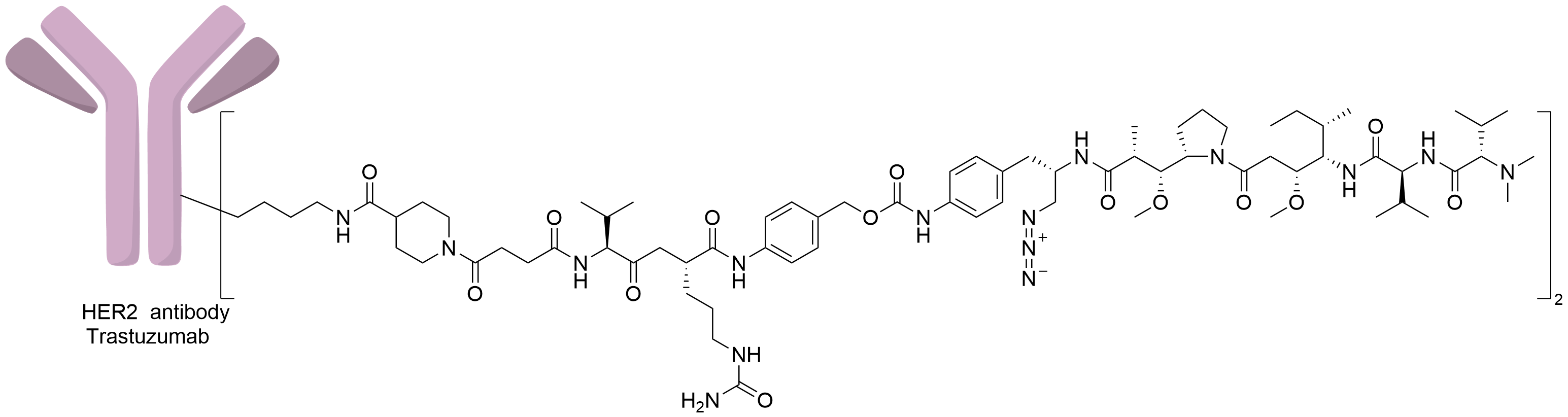
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Duostatin 5
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
K-lock-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
25.92%
|
|||
| Patients Enrolled |
Locally advanced/metastatic solid tumors expressing human epidermal growth factor receptor 2 (HER2) or are HER2 amplified.
|
||||
| Administration Dosage |
0.30, 1.20, 3.60, 4.80 mg/kg, every 3 weeks from date of enrollment until the date of first documented progression or date of death from any cause, whichever came first, assessed up to 24 months.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03602079 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1-2, FIH Study of A166 in locally advanced/metastatic solid tumors expressing human epidermal growth factor receptor 2 (HER2) or are HER2 amplified that did not respond or stopped responding to approved therapies. | ||||
| Primary Endpoint |
Overall incidence of ophthalmic toxicities in the 3.60 mg/kg cohort was 80% and in the 4.80 mg/kg cohort it was 83.00%. Responses were seen only at the dose levels of 3.60 mg/kg and 4.80 mg/kg. Among the 27 patients evaluable for efficacy,best response was progression of disease in 11 patients (40.74%), stable disease in 9 patients (33.33%) and partial response in 7 patients (25.92%),for the total disease control rate of 59%.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
73.90% (4.8 mg/kg)
68.60% (6.0 mg/kg) |
|||
| Patients Enrolled |
Patients with HER2-expressing advanced solid tumours.
|
||||
| Administration Dosage |
0.10, 0.30, 0.60, 1.20, 2.40, 3.60, 4.80 or 6.00 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05311397 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of A166 in patients with unresectable, locally advanced or metastatic HER2-expressing solid tumors (KL166-I-01-CTP). | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05346328 | Clinical Status | Phase 2 | ||
| Clinical Description | An open-clinical trial phase , injection of A166 for HER2-positive patients with refractory unresectable locally advanced or metastatic breast cancer KL166-2S-001. | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03602079 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1-2, FIH study of A166 in locally advanced/metastatic solid tumors expressing human epidermal growth factor receptor 2 (HER2) or are HER2 amplified that did not respond or stopped responding to approved therapies. | ||||
References
