Payload Information
General Information of This Payload
| Payload ID | PAY0EQOWS |
|||||
|---|---|---|---|---|---|---|
| Name | Duostatin 5 |
|||||
| Synonyms |
Duostatin 5; CE421MMY47; 2124210-34-8; UNII-CE421MMY47; DUO-5; SCHEMBL20846018; EX-A5872; HY-145149; CS-0356744
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Microtubule (MT) | ||||||
| Structure |
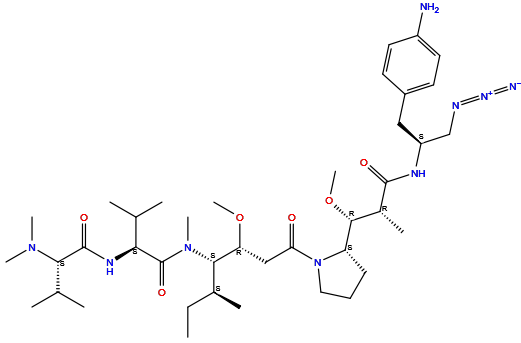
|
|||||
| Formula | C40H69N9O6 |
|||||
| Isosmiles | [H]c1c([H])c(C([H])([H])[C@]([H])(N([H])C(=O)[C@]([H])(C([H])([H])[H])[C@@]([H])(OC([H])([H])[H])[C@@]2([H])N(C(=O)C([H])([H])[C@@]([H])(OC([H])([H])[H])[C@@]([H])(N(C(=O)[C@@]([H])(N([H])C(=O)[C@@]([H])(N(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])[C@@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C2([H])[H])C([H])([H])N=[N+]=[N-])c([H])c([H])c1N([H])[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C40H69N9O6/c1-13-26(6)36(48(10)40(53)34(24(2)3)45-39(52)35(25(4)5)47(8)9)32(54-11)22-33(50)49-20-14-15-31(49)37(55-12)27(7)38(51)44-30(23-43-46-42)21-28-16-18-29(41)19-17-28/h16-19,24-27,30-32,34-37H,13-15,20-23,41H2,1-12H3,(H,44,51)(H,45,52)/t26-,27+,30-,31-,32+,34-,35-,36-,37+/m0/s1
|
|||||
| InChIKey |
QIHHTRNUHMRKQK-MNFMTBGBSA-N
|
|||||
| IUPAC Name |
(2S)-N-[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(2S)-1-(4-aminophenyl)-3-azidopropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-2-[[(2S)-2-(dimethylamino)-3-methylbutanoyl]amino]-N,3-dimethylbutanamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
772.049 |
Polar area |
195.3 |
||
Complexity |
771.5370809 |
xlogp Value |
4.2537 |
|||
Heavy Count |
55 |
Rot Bonds |
35 |
|||
Hbond acc |
9 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Trastuzumab botidotin [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
25.92%
|
|||
| Patients Enrolled |
Locally advanced/metastatic solid tumors expressing human epidermal growth factor receptor 2 (HER2) or are HER2 amplified.
|
||||
| Administration Dosage |
0.30, 1.20, 3.60, 4.80 mg/kg, every 3 weeks from date of enrollment until the date of first documented progression or date of death from any cause, whichever came first, assessed up to 24 months.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03602079 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1-2, FIH Study of A166 in locally advanced/metastatic solid tumors expressing human epidermal growth factor receptor 2 (HER2) or are HER2 amplified that did not respond or stopped responding to approved therapies.
|
||||
| Primary Endpoint |
Overall incidence of ophthalmic toxicities in the 3.60 mg/kg cohort was 80% and in the 4.80 mg/kg cohort it was 83.00%. Responses were seen only at the dose levels of 3.60 mg/kg and 4.80 mg/kg. Among the 27 patients evaluable for efficacy,best response was progression of disease in 11 patients (40.74%), stable disease in 9 patients (33.33%) and partial response in 7 patients (25.92%),for the total disease control rate of 59%.
Click to Show/Hide
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
73.90% (4.8 mg/kg)
68.60% (6.0 mg/kg) |
|||
| Patients Enrolled |
Patients with HER2-expressing advanced solid tumours.
|
||||
| Administration Dosage |
0.10, 0.30, 0.60, 1.20, 2.40, 3.60, 4.80 or 6.00 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05311397 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 study to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of A166 in patients with unresectable, locally advanced or metastatic HER2-expressing solid tumors (KL166-I-01-CTP).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05346328 | Phase Status | Phase 2 | ||
| Clinical Description |
An open-clinical trial phase , injection of A166 for HER2-positive patients with refractory unresectable locally advanced or metastatic breast cancer KL166-2S-001.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03602079 | Phase Status | Phase 1/2 | ||
| Clinical Description |
A phase 1-2, FIH study of A166 in locally advanced/metastatic solid tumors expressing human epidermal growth factor receptor 2 (HER2) or are HER2 amplified that did not respond or stopped responding to approved therapies.
|
||||
AMT-151 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05498597 | Phase Status | Phase 1 | ||
| Clinical Description |
First-in-human, phase 1 study of AMT-151, an anti-folate receptor alpha antibody-drug conjugate, in patients with selected advanced solid tumours.
|
||||
References
