Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0SZERO
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Lonigutamab ugodotin
|
|||||
| Synonyms |
F50085-hz208F2-4-F556311; Lonigutamab ugodotin; W 0101; W-0101; W0101
Click to Show/Hide
|
|||||
| Organization |
Pierre Fabre SA,
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
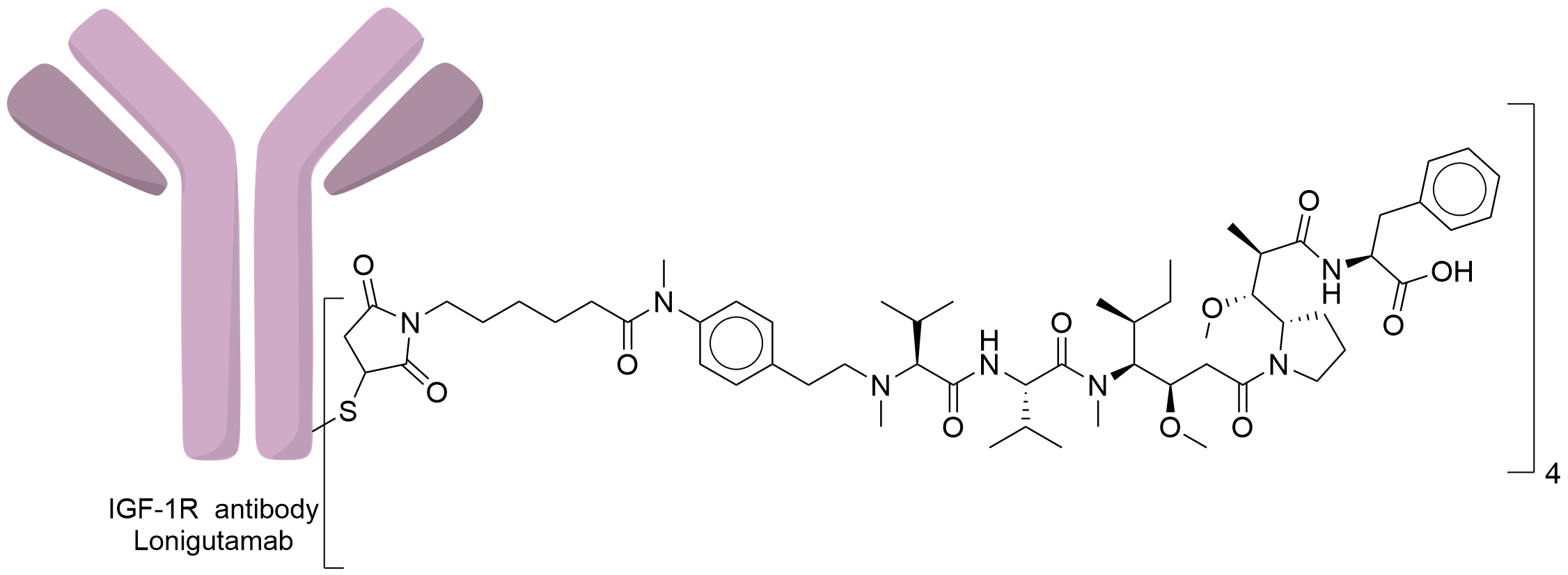
|
|||||
| Antibody Name |
Lonigutamab
|
Antibody Info | ||||
| Antigen Name |
Insulin-like growth factor 1 receptor (IGF1R)
|
Antigen Info | ||||
| Payload Name |
F554443
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
ugodotin
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03316638 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Phase 1/2 open label dose escalation and dose expansion study of intravenous infusion of W0101, an antibody-drug conjugate, in patients with advanced or metastatic solid tumors. International, multicenter, open label study. | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% | Low IGF1 expression (IGF1+) | ||
| Method Description |
For the lung cancer models, 7-week-old female athymic nude mice (Envigo) were engrafted subcutaneously with 7 x106 SBC5 cells and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
|
||||
| In Vitro Model | Lung small cell carcinoma | SBC-5 cells | CVCL_1679 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.00% | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
For the lung cancer models, 7-week-old female athymic nude mice (Envigo) were engrafted subcutaneously with 7 x106 NCI-H2122 cells and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2122 cells | CVCL_1531 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% | High IGF1 expression (IGF1+++) | ||
| Method Description |
For the breast cancer model, 7-week-old female Swiss nude mice (Charles River Laboratories) were engrafted subcutaneously with 5 x106 MCF-7 cells 1 day after subcutaneous implantation of 0.72 mg 17-estradiol 60-day releasing pellets (Innovative Research of America) and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
For the ovarian cancer model, 7-week-old female SCID mice (Charles River Laboratories) were engrafted subcutaneously with 10 x106 CaoV3 cells and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2122 cells | CVCL_1531 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.01 nM | High IGF1 expression (IGF1+++) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.63 nM | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2122 cells | CVCL_1531 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Low IGF1 expression (IGF1+) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | SBC-5 cells | CVCL_1679 | ||
References
