Payload Information
General Information of This Payload
| Payload ID | PAY0HXYLH |
|||||
|---|---|---|---|---|---|---|
| Name | F554443 |
|||||
| Synonyms |
F554443
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
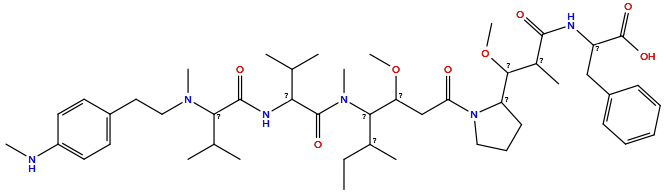
|
|||||
| Formula | C48H76N6O8 |
|||||
| Isosmiles | CCC(C)C(C(CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(Cc1ccccc1)C(=O)O)OC)N(C)C(=O)C(NC(=O)C(C(C)C)N(C)CCc1ccc(NC)cc1)C(C)C |
|||||
| InChI |
InChI=1S/C48H76N6O8/c1-13-32(6)43(53(10)47(58)41(30(2)3)51-46(57)42(31(4)5)52(9)27-25-34-21-23-36(49-8)24-22-34)39(61-11)29-40(55)54-26-17-20-38(54)44(62-12)33(7)45(56)50-37(48(59)60)28-35-18-15-14-16-19-35/h14-16,18-19,21-24,30-33,37-39,41-44,49H,13,17,20,25-29H2,1-12H3,(H,50,56)(H,51,57)(H,59,60)
|
|||||
| InChIKey |
PYQZMUCAWOGBMY-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
865.17 |
Polar area |
169.85 |
||
Complexity |
864.5724634 |
xlogp Value |
5.1004 |
|||
Heavy Count |
62 |
Rot Bonds |
25 |
|||
Hbond acc |
9 |
Hbond Donor |
4 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Lonigutamab ugodotin [Phase 1/2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03316638 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1/2 open label dose escalation and dose expansion study of intravenous infusion of W0101, an antibody-drug conjugate, in patients with advanced or metastatic solid tumors. International, multicenter, open label study.
|
||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 0.00% | Low IGF1 expression (IGF1+) | ||
| Method Description |
For the lung cancer models, 7-week-old female athymic nude mice (Envigo) were engrafted subcutaneously with 7 x106 SBC5 cells and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
|
||||
| In Vitro Model | Lung small cell carcinoma | SBC-5 cells | CVCL_1679 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 80.00% | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
For the lung cancer models, 7-week-old female athymic nude mice (Envigo) were engrafted subcutaneously with 7 x106 NCI-H2122 cells and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2122 cells | CVCL_1531 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% | High IGF1 expression (IGF1+++) | ||
| Method Description |
For the breast cancer model, 7-week-old female Swiss nude mice (Charles River Laboratories) were engrafted subcutaneously with 5 x106 MCF-7 cells 1 day after subcutaneous implantation of 0.72 mg 17-estradiol 60-day releasing pellets (Innovative Research of America) and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
For the ovarian cancer model, 7-week-old female SCID mice (Charles River Laboratories) were engrafted subcutaneously with 10 x106 CaoV3 cells and treated with W0101 or isotype control ADC at 3 mg/kg every 4 days for 4 cycles.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2122 cells | CVCL_1531 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
High IGF1 expression (IGF1+++) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.63 nM
|
Moderate IGF1 expression (IGF1++) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H2122 cells | CVCL_1531 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 nM | Moderate IGF1 expression (IGF1++) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Ovarian serous adenocarcinoma | Caov-3 cells | CVCL_0201 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 nM | Low IGF1 expression (IGF1+) | ||
| Method Description |
Tumor and normal cells were plated in 96-well flat bottomed microplates (100 L/well) in cell culture medium and incubated overnight at 37°C in 5% CO2. The next day, increasing concentrations of W0101 or isotype control ADC (010 ug/mL) were added into 3 replicate wells containing cells (10 L/well). Plates were incubated for 6 days at 37°C in 5% CO2. Cell viability was determined by measuring ATP using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). Luminescence was read using a Multimode Microplate Reader (Mithras LB940, Berthold Technologies). The percentage cell viability was calculated for each concentration considering 0 ug/mL ADC as 100% viability. The IC50 was calculated using Prism software. Three independent experiments were performed.
Click to Show/Hide
|
||||
| In Vitro Model | Lung small cell carcinoma | SBC-5 cells | CVCL_1679 | ||
References
