Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0QRDUT
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
AGS-16C3F
|
|||||
| Synonyms |
AGS 16C3F; AGS-16C3F; AGS-16F; AGS-16M8F; AGS16C3F; Anti-ENPP3-MMAF
Click to Show/Hide
|
|||||
| Organization |
Agensys, Inc.; Astellas Pharma, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
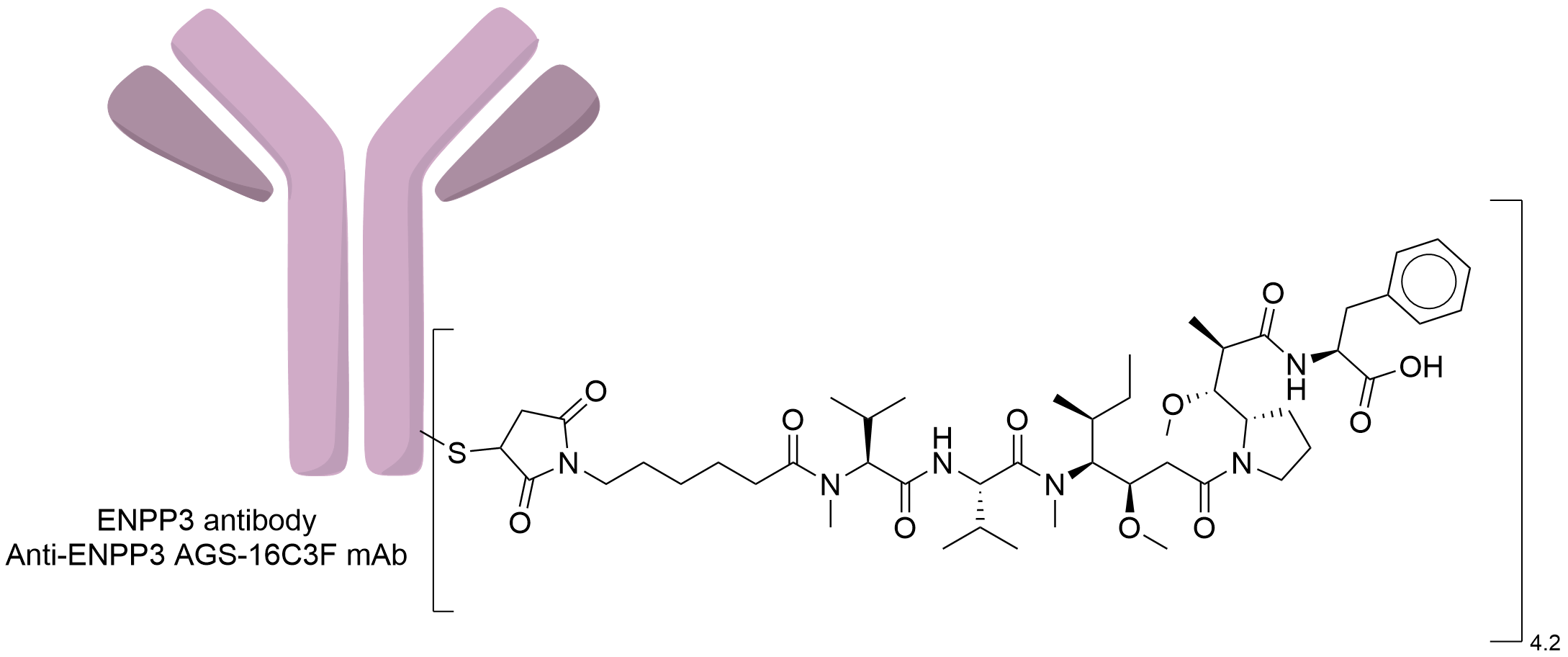
|
|||||
| Antibody Name |
Anti-ENPP3 AGS-16C3F mAb
|
Antibody Info | ||||
| Antigen Name |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3 (ENPP3)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin F
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
mafodotin
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) | 23.08% | High ENPP3 expression (ENPP3+++) | ||
| Patients Enrolled |
Metastatic renal cell carcinoma (MRCC), Eastern Cooperative Oncology Group (ECOG) performance status 1, adequate organ and bone marrow function.
|
||||
| Administration Dosage |
AGS-16M8F was administered intravenously every 3 weeks at 5 dose levels ranging from 0.60 to 4.80 mg/kg until unacceptable toxicity or progression. A second study with AGS-16C3F started with the AGS-16M8F bridging dose of 4.80 mg/kg given every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT01672775 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open label, multi-center study to assess the safety, pharmacokinetics and effectiveness of AGS-16C3F monotherapy in subjects with renal cell carcinoma (RCC) of clear cell or papillary histology. | ||||
| Primary Endpoint |
In the AGS-16C3F study (n = 34),the MTD was 3.60 mg/kg,but this was not tolerated. The 1.80 mg/kg dose was determined to be safe and was associated antitumor response.
|
||||
| Other Endpoint |
3 subjects at 1.80 mg/kg achieved durable PR (3/13, 23.08%). The disease control rate at 1.80 mg/kg was 92.30% (N=12/13). The disease control rate for the entire study was 58.82% (N=20/34).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 7.50% (AGS16C3F), 18.20% (axitinib) | Moderate ENPP3 expression (ENPP3++) | ||
| Patients Enrolled |
Advanced renal cell carcinoma (RCC).
|
||||
| Administration Dosage |
Intravenous AGS-16C3F 1.80 mg/kg every 3 weeks or oral axitinib 5 mg twice daily (starting dose).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02639182 | Clinical Status | Phase 2 | ||
| Clinical Description | A multi-center, open label, randomized phase 2 study of AGS-16C3F vs. axitinib in metastatic renal cell carcinoma. | ||||
| Primary Endpoint |
Median PFS=2.90 months (95% CI,2.00-4.00) for AGS16C3F,Median PFS=5.7 months (95% CI,5.30-9.10) for axitinib.
|
||||
| Other Endpoint |
Disease Control Rate (DCR)=13.40% (95% CI,6.3-24.0) for AGS16C3F, Disease Control Rate (DCR)=22.70% (95% CI,13.30-34.70) for axitinib. Median duration of Response (mDoR)=6.80 months (95% CI,3.80-18.40) for AGS16C3F, Median duration of Response (mDoR)=6.7 months (95% CI,1.80-9.20) for axitinib. Objective Response Rate (ORR)=7.50% (95% CI,2.50-16.60) for AGS16C3F, Objective Response Rate (ORR)=18.20% (95% CI,9.80-29.60) for axitinib. Median Overall Survival (mOS)=13.10 months for AGS16C3F, Median Overall Survival (mOS)=15.40 months for axitinib.
Click to Show/Hide
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.10 nM
|
|||
| Method Description |
Cells were incubated in triplicate in medium containing AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF (0 [Control],0.001,0.003,0.008,0.02,0.07,0.21,0.62,1.82,5.57,16.67,50,150,450,and 1350 nM) in a 5% CO2 incubator at 37°C for 96 hours. IC50 values at day 5 for AGS-16C3F were calculated for each cell line.
|
||||
| In Vitro Model | Normal | ROSA KIT D816V cells | CVCL_5G50 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 2.73 nM | Moderate ENPP3 expression (ENPP3++) | ||
| Method Description |
Cells were incubated in triplicate in medium containing AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF (0 [Control],0.001,0.003,0.008,0.02,0.07,0.21,0.62,1.82,5.57,16.67,50,150,450,and 1350 nM) in a 5% CO2 incubator at 37°C for 96 hours. IC50 values at day 5 for AGS-16C3F were calculated for each cell line.
|
||||
| In Vitro Model | Mast-cell sarcoma | ROSA KIT D816V Gluc cells | Homo sapiens | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 109.90 nM | High ENPP3 expression (ENPP3+++) | ||
| Method Description |
Cells were incubated in triplicate in medium containing AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF (0 [Control],0.001,0.003,0.008,0.02,0.07,0.21,0.62,1.82,5.57,16.67,50,150,450,and 1350 nM) in a 5% CO2 incubator at 37°C for 96 hours. IC50 values at day 5 for AGS-16C3F were calculated for each cell line.
|
||||
| In Vitro Model | Mast cell leukemia | HMC-1.1 cells | CVCL_H206 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
146.50 nM
|
|||
| Method Description |
Cells were incubated in triplicate in medium containing AGS-16C3F or cHmLYS-1c3.G2k-mcMMAF (0 [Control],0.001,0.003,0.008,0.02,0.07,0.21,0.62,1.82,5.57,16.67,50,150,450,and 1350 nM) in a 5% CO2 incubator at 37°C for 96 hours. IC50 values at day 5 for AGS-16C3F were calculated for each cell line.
|
||||
| In Vitro Model | Mast cell leukemia | HMC-1.2 cells | CVCL_H205 | ||
References
