Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0QMQFL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
chmAb-C B7-H3-ADC
|
|||||
| Synonyms |
chmAb-C B7-H3 ADC
Click to Show/Hide
|
|||||
| Organization |
Macrogenics, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 6 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
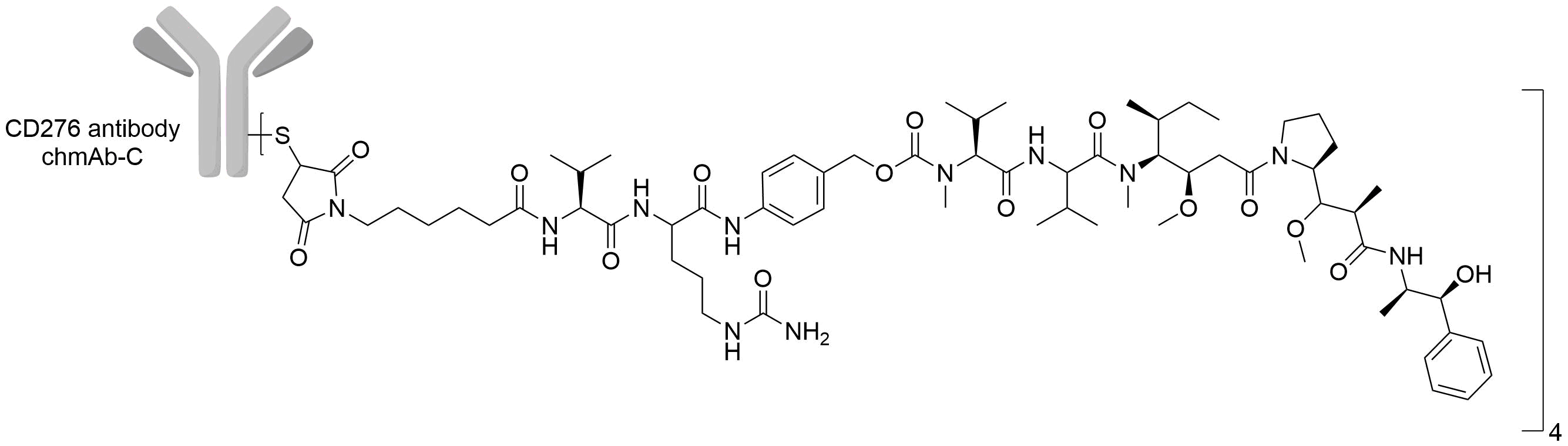
|
|||||
| Antibody Name |
chmAb-C
|
Antibody Info | ||||
| Antigen Name |
CD276 antigen (CD276)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 13.21% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted PA-1 ovarian cancer cells, and show responsiveness against the PA-1 tumor cells. The dose was 1 mg/kg on day 30.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 14.62% (Day 43) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted Calu-6 lung cancer cells, and show responsiveness against the Calu-6 tumor cells. The dose was 3 mg/kg on day 30.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 25.99% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted PA-1 ovarian cancer cells, and show responsiveness against the PA-1 tumor cells. The dose was 3 mg/kg on day 30.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.46% (Day 49) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted A375.52 melanoma cells, and show responsiveness against the A375.52 tumor cells. The dose was 1 mg/kg on day 30.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 30.91% (Day 91) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted NCI-H1703 non-small cell lung cancer cells, and show responsiveness against the NCI-H1703 tumor cells. The dose was 1 mg/kg on day 30.
|
||||
| In Vivo Model | NCI-H1703 CDX model | ||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 32.04% (Day 43) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted Calu-6 lung cancer cells, and show responsiveness against the Calu-6 tumor cells. The dose was 1 mg/kg on day 30.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.42% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted PA-1 ovarian cancer cells, and show responsiveness against the PA-1 tumor cells. The dose was 10 mg/kg on day 30.
|
||||
| In Vivo Model | PA-1 CDX model | ||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 69.12% (Day 91) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted NCI-H1703 non-small cell lung cancer cells, and show responsiveness against the NCI-H1703 tumor cells. The dose was 10 mg/kg on day 30.
|
||||
| In Vivo Model | NCI-H1703 CDX model | ||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.78% (Day 49) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted A375.52 melanoma cells, and show responsiveness against the A375.52 tumor cells. The dose was 3 mg/kg on day 30.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.78% (Day 91) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted NCI-H1703 non-small cell lung cancer cells, and show responsiveness against the NCI-H1703 tumor cells. The dose was 3 mg/kg on day 30.
|
||||
| In Vivo Model | NCI-H1703 CDX model | ||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.90% (Day 77) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted MDAMB-468 breast cancer tumor cells, and show responsiveness against the MDA-MB-468 tumor cells. The dose was 10 mg/kg on day 30.
|
||||
| In Vivo Model | MDA-MB-468 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.77% (Day 49) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted A375.52 melanoma cells, and show responsiveness against the A375.52 tumor cells. The dose was 10 mg/kg on day 30.
|
||||
| In Vivo Model | A375.52 CDX model | ||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 97.44% (Day 70) | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
The results of this study with respect to mammary fat pad implanted Calu-6 lung cancer cells, and show responsiveness against the Calu-6 tumor cells. The dose was 10 mg/kg on day 30.
|
||||
| In Vivo Model | Calu-6 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 16.00 pM | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 30.00 pM | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-6 cells | CVCL_0236 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 43.00 pM | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H1703 cells | CVCL_1490 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.11 nM | High CD276 expression (CD276 +++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Neoplasm | Hs 700T cells | CVCL_0858 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.12 nM | High CD276 expression (CD276 +++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.20 nM | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.27 nM | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Amelanotic melanoma | A375.S2 cells | CVCL_0136 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.41 nM | Moderate CD276 expression (CD276 ++) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Ovarian mixed germ cell tumor | PA-1 cells | CVCL_0479 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.47 nM | Low CD276 expression (CD276 +) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | Prostate carcinoma | DU145 cells | CVCL_0105 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100 nM | Negative CD276 expression (CD276 -) | ||
| Method Description |
Briefly, B7-H3-ADCs and controls are diluted and plated intomicrotiter plates, 5000 cells are added to each well and incubated at 37°C for 4-7 daysAlamar Blue Reagent is added to the plates andread according to the manufacturer's protocol. The number of antibody binding sites presenton these cells was determined using a Bangs QFACSTM Kit.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
References
