Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0QMIBK
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
FOR-46
|
|||||
| Synonyms |
Anti-CD46 antibody drug conjugate; FOR 46; FOR-46; FOR46; FG-3246; FG 3246; FG3246
Click to Show/Hide
|
|||||
| Organization |
University of California San Diego; Fortis Therapeutics, Inc.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.7
|
|||||
| Structure |
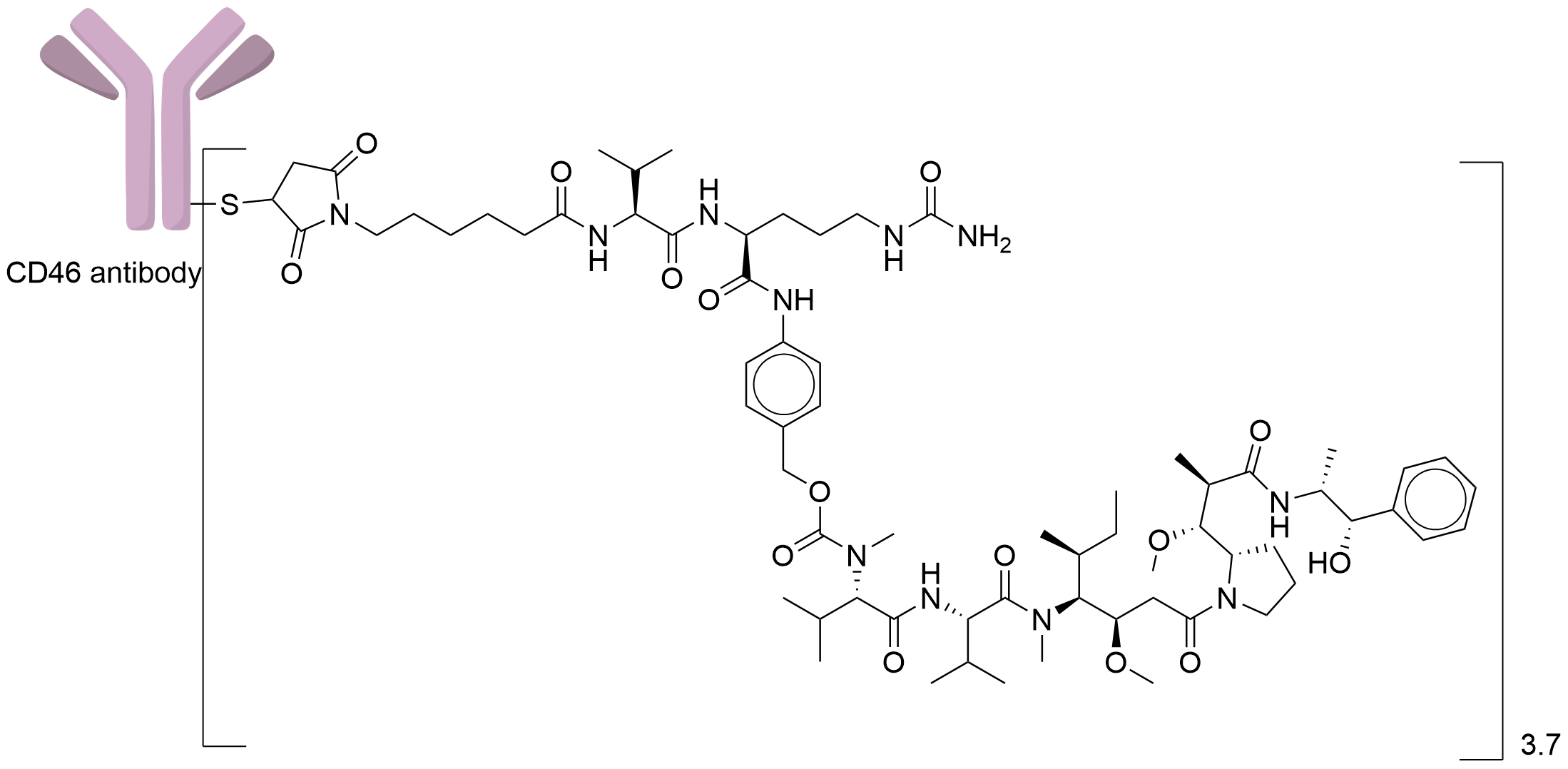
|
|||||
| Antibody Name |
Anti-CD46 fully human Anti-ody
|
Antibody Info | ||||
| Antigen Name |
Membrane cofactor protein (CD46)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
22.20%
|
|||
| Patients Enrolled |
Metastatic castration-resistant prostate cancer (CRPC).
|
||||
| Administration Dosage |
Intravenous FOR46 on day 1 of every 21-day cycle. Dose levels ranged from 0.10 mg/kg. the starting dose level, to 3.00 mg/kg. The trial established 2.70 mg/kg to be the maximum tolerated dose (MTD) and the recommended phase 2 dose (RP2D) of the agent.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03575819 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of FOR46 administered every 21 days in patients with metastatic castration-resistant prostate cancer (mCRPC). | ||||
| Primary Endpoint |
MtD and phase 1b dose=2.70 mg/kg.
|
||||
| Other Endpoint |
18 pts had measurable lesions; 8 of 18 (44.44%) had tumor regression, with 4 (22.22%) confirmed partial responses (PR). The median duration of response is > 14 wks (range 9 -31+ weeks).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05011188 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1b/2 study of FOR46 in combination with enzalutamide in patients with metastatic castration resistant prostate cancer. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03650491 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study of FOR46 administered every 21 days in patients with relapsed or refractory multiple myeloma (RRMM). | ||||
References
