Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0PXQWR
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Depatuxizumab mafodotin
|
|||||
| Synonyms |
ABT-414; ABT-414/806; Depatux-M; Depatuxizumab mafodotin
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3.8
|
|||||
| Structure |
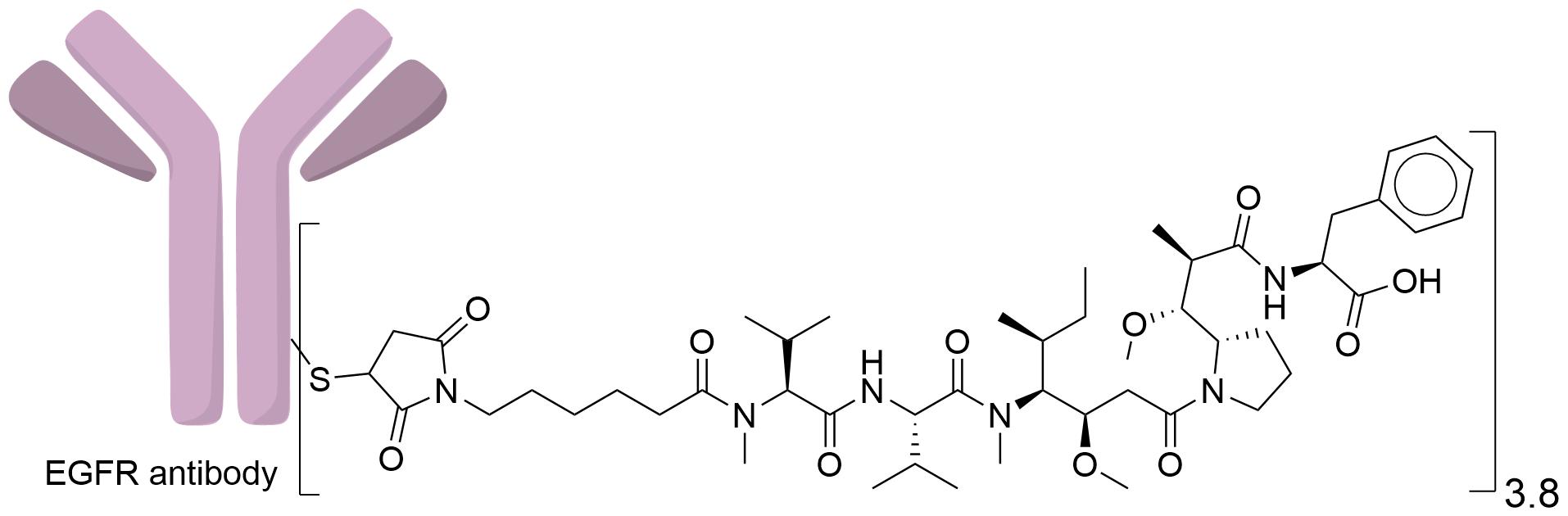
|
|||||
| Antibody Name |
Depatuxizumab
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin F
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
mafodotin
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median progression-free survival (mPFS) | 8.00 (depatux-m group); 6.30 (placebo group) | High EGFR expression (EGFR +++) | ||
| Patients Enrolled |
EGFR-amp newly diagnosed GBM were randomized 1:1 to radiotherapy, temozolomide, and depatux-m/placebo.
|
||||
| Administration Dosage |
Depatux-m was dosed at 2.0 mg/kg during RT, then 1.25 mg/kg thereafter on days 1 and 15/28, 19,21 and allowed to continue until disease progression.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median Overall Survival (mOS) | 18.90 (depatux-m group); 18.70(placebo group) | High EGFR expression (EGFR +++) | ||
| Patients Enrolled |
EGFR-amp newly diagnosed GBM were randomized 1:1 to radiotherapy, temozolomide, and depatux-m/placebo.
|
||||
| Administration Dosage |
Depatux-m was dosed at 2.0 mg/kg during RT, then 1.25 mg/kg thereafter on days 1 and 15/2819, 21 and allowed to continue until disease progression.
|
||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.50% (Day 40) | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
To establish xenografts, 2 x 106 MSTO-211H cells mixed with 75-uL Matrigel were injected subcutaneously in the right flank of 5 to 6-week-old female BALB/c nu/nu miceFor the MSTO-211H study, mice received either ABT-414, ABBV-221 or ADC control (3 mg/kg) every 4 days.
|
||||
| In Vivo Model | MSTO-211H CDX model | ||||
| In Vitro Model | Pleural biphasic mesothelioma | MSTO-211H cells | CVCL_1430 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 10.00 ug/mL - 35.00 ug/mL | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural biphasic mesothelioma | MSTO-211H cells | CVCL_1430 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 10.00 ug/mL - 35.00 ug/mL | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural mesothelioma | NCI-H28 cells | CVCL_1555 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 10.00 ug/mL - 35.00 ug/mL | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural mesothelioma | NCI-H2052 cells | CVCL_1518 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 10.00 ug/mL - 35.00 ug/mL | Positive EGFR expression (EGFR+++/++) | ||
| Method Description |
Cells lines were plated at 1,000-3,000 cells per well in complete growth medium containing 10% FCS in 96-well plates and allowed to adhere overnight.
|
||||
| In Vitro Model | Pleural mesothelioma | NCI-H2052 cells | CVCL_1518 | ||
References
