Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0PNJIT
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Tisotumab vedotin
|
|||||
| Brand Name |
Tivdak
|
|||||
| Synonyms |
HuMax-TF-ADC;Tisotumab vedotin;GCT1015-04;IGG1-1015-011-1006;TF-011-MMAE;WHO 10148;Humax TF ADC;HuMax-TF;HuMax-TF-ADC
Click to Show/Hide
|
|||||
| Organization |
Seagen Inc.; Genmab A/S; Zai Lab Ltd; Rps Medical Technology (Beijing) Co., Ltd.
|
|||||
| Drug Status |
Approved (FDA): Sep 22, 2021
|
|||||
| Indication |
In total 11 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
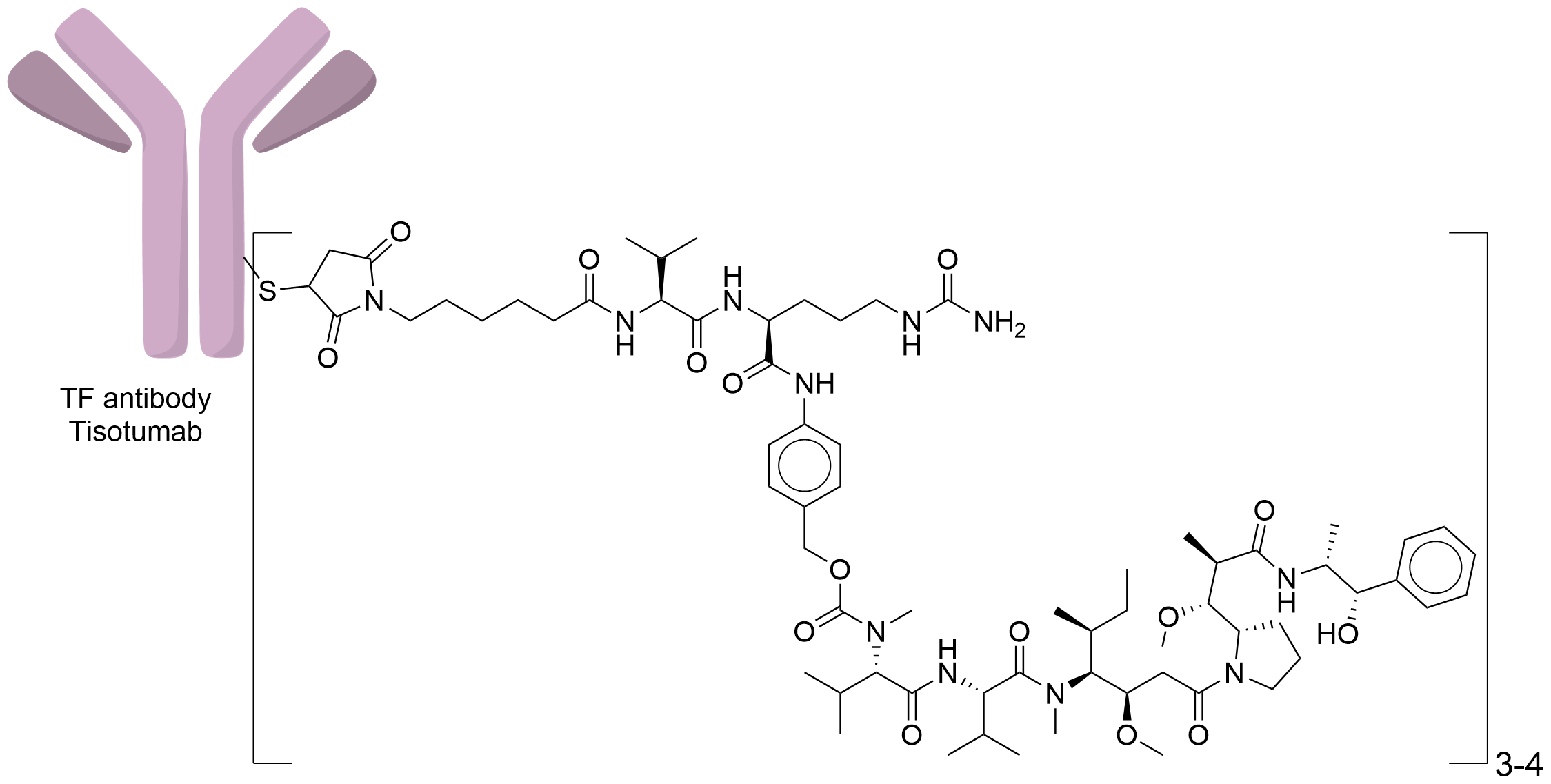
|
|||||
| Antibody Name |
Tisotumab
|
Antibody Info | ||||
| Antigen Name |
Tissue factor (F3)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Combination Type |
Vedotin
|
|||||
| Absorption |
For tisotumab vedotin-tftv (2 mg/kg), mean Cmax=40.80±8.12 (ug/mL), mean AUC=57.50±13.40 (day x ug/mL) and steady-state concentrations were reached after one treatment cycle. For unconjugated MMAE, mean Cmax=5.91±4.20 (ug/mL), mean AUC=50.00±35.80 (day x ug/mL) and steady-state concentrations were reached after one treatment cycle.
|
|||||
| Distribution |
The tisotumab vedotin-tftv steady-state volume of distribution is 7.83 (%CV: 19.1) L. Plasma protein binding of MMAE ranged from 68% to 82% in vitro.
|
|||||
| Metabolism |
Tisotumab vedotin-tftv most likely undergoes catabolism to form small peptides, amino acids, unconjugated MMAE, and unconjugated MMAE-related catabolites. Via proteolytic cleavage, tisotumab vedotin-tftv releases unconjugated MMAE, which is primarily metabolized by CYP3A4 in vitro. The median terminal half-life of tisotumab vedotin-tftv was 4.04 (range: 2.26-7.25) days. The median terminal half-life of unconjugated MMAE was 2.56 (range: 1.81-4.10) days.
|
|||||
| Elimination |
Tisotumab vedotin-tftv's excretion isn't fully understood. Another MMAE-linked drug showed 17% fecal and 6% urinary excretion in a week, mostly as the original drug. Tisotumab vedotin-tftv is likely to have a similar excretion pattern. The linear clearance of tisotumab vedotin-tftv was 1.54 (%CV: 28.8) L/day. The linear clearance of unconjugated MMAE was 45.9 (%CV: 61.1) L/day.
|
|||||
| Toxicity |
Tisotumab vedotin is associated with a risk for ocular toxicity. In clinical trials, ocular adverse reactions occurred in 60% of patients with cervical cancer. The most common reactions were conjunctival adverse reactions (40%), dry eye (29%), corneal adverse reactions (21%), and blepharitis (8%). More severe reactions included ulcerative keratitis, ulcerative keratitis with perforation requiring corneal transplantation, and symblepharon in patients with other tumor types.
|
|||||
| Special Approval(s) |
Accelerated approval(FDA)
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) | 40.00% | Moderate Tissue factor expression (TF++; IHC H-score=155) | ||
| Patients Enrolled |
Ovary Cancer; Cervix Cancer; Endometrium Cancer; Bladder Cancer; Prostate Cancer; Esophagus Cancer; Lung Cancer, Nonsmall Cell; Squamous Cell Carcinoma of the Head and Neck.
|
||||
| Administration Dosage |
Once every 3 weeks intravenous (IV).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03245736 | Clinical Status | Phase 2 | ||
| Clinical Description | A multi-center, open-label trial investigating the efficacy and safety of continued treatment with tisotumab vedotin in patients with solid tumors known to express tissue factor. | ||||
| Primary Endpoint |
Number of participants who experienced a treatment emergent adverse event (TEAE): 5/5 Participants (100%).
|
||||
| Other Endpoint |
Partial Response Rate=2/5 (40.00%), Stable Disease Rate=2/5(40.00%), Progressive Disease Rate=1/5 (20.00%), Increased Cancer Antigen (CA 125) Levels Rate=1/2 (50.00%).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 24.00% | High Tissue factor expression (TF+++; IHC H-score=250) | ||
| Patients Enrolled |
Recurrent or metastatic squamous cell, adenocarcinoma, or adenosquamous cervical cancer; disease progression on or after doublet chemotherapy with bevacizumab (if eligible by local standards); who had received two or fewer previous systemic regimens for recurrent or metastatic disease; had measurable disease based on Response Evaluation Criteria in Solid Tumors (RECIST; version 11); and had an Eastern Cooperative Oncology Group performance status of 0 or 1.
Click to Show/Hide
|
||||
| Administration Dosage |
20 mg/kg (up to a maximum of 200 mg) intravenously once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03438396 | Clinical Status | Phase 2 | ||
| Clinical Description | A Single arm, multicenter, international trial of tisotumab vedotin (HuMax-TF-ADC) in previously treated, recurrent or metastatic cervical cancer. | ||||
| Primary Endpoint |
Objective response rate=24.00% (95% CI 16.00%-33.00%), comprising 7 (7.00%) complete responses and 17 (17.00%) partial responses, Disease Control Rate (DCR)=72.00%.
|
||||
| Other Endpoint |
Median duration of response=8.30 months, 62.00% (95% CI 3.70-8.00) of patients > 6months,median progression-free survival = 4.20 months (95% Cl 3.00-4.40), median overall survival = 12.10 months (95% Cl, 9.60-13.90).
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 38.24% (second- or third-line group) | Low Tissue factor expression (TF+; <7,000 TF molecules/cell) | ||
| Patients Enrolled |
Recurrent/metastatic cervical cancer (r/mCC).
|
||||
| Administration Dosage |
20 mg/kg + pembro 200 mg IV Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03786081 | Clinical Status | Phase 1b/2 | ||
| Clinical Description | A phase 1b/2 open-label trial of tisotumab vedotin (HuMax-TF-ADC) monotherapy and in combination with other agents in subjects with recurrent or stage IVB cervical cancer. | ||||
| Primary Endpoint |
In the second- or third-line group,objective response rate=38.24% (95% Cl 22.00-56.00), comprising 2 (5.88%) complete responses and 11 (32.35%) partial responses.
|
||||
| Other Endpoint |
In the second- or third-line group,median duration of response was 13.80 months, median progression-free survival was 5.60 months.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 54.55% (first-line treatment group) | High Tissue factor expression (TF+++; >300,000 TF molecules/cell) | ||
| Patients Enrolled |
Recurrent/metastatic cervical cancer (r/mCC).
|
||||
| Administration Dosage |
20 mg/kg + carbo AUC 5 IV every 3 weeks (Q3W).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03786081 | Clinical Status | Phase 1b/2 | ||
| Clinical Description | A phase 1b/2 open-label trial of tisotumab vedotin (HuMax-TF-ADC) monotherapy and in combination with other agents in subjects with recurrent or stage IVB cervical cancer. | ||||
| Primary Endpoint |
In the first-line treatment group, objective response rate= 54.55%, comprising 4 (12.12%) complete responses and 14 (42.42%) partial responses.
|
||||
| Other Endpoint |
In the first-line treatment group, median duration of response=83 months,median progression-free survival was 95 months.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 15.65% (all patients), 26.67% (Bladder cancer), 26.47% (Cervical cancer), 7.14% (Endometrial cancer), 13.33% (Oesophageal cancer), 13.33% (NSCLC), 13.89% (Ovarian cancer) | Low Tissue factor expression (TF+; <15,000 TF molecules/cell) | ||
| Patients Enrolled |
Relapsed, advanced, or metastatic cancer of the ovary, cervix, endometrium, bladder, prostate, oesophagus, squamous cell carcinoma of the head and neck or non-small-cell lung cancer; an Eastern Cooperative Oncology Group performance status of 0-1; and had relapsed after or were not eligible to receive the available standard of care.
|
||||
| Administration Dosage |
0.3 and 2.2 mg/kg intravenously once every 3 weeks in a traditional 3+3 design.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02001623 | Clinical Status | Phase 1/2 | ||
| Clinical Description | First-in-human, dose-escalating safety study of tissue factor specific antibody drug conjugate tisotumab vedotin (HuMax TF ADC) in patients with locally advanced and/or metastatic solid tumors known to express tissue factor. | ||||
| Primary Endpoint |
OrR of all patients=15.65% (23/147, 95% Cl 10.20-22.00), ORR of Bladder cancer=26.67% (4/15, 95% Cl 7.80-55.10), ORR of Cervical cancer=26.47% (9/34, 95% Cl 12.90-44.40), ORR of Endometrial cancer=7.14% (1/14, 95% Cl 0.20-33.90), ORR of Oesophageal cancer=13.33% (2/15, 95% Cl 1.70-40.50), ORR of NSCLC=13.33% (2/15, 95% Cl 1.70-40.50), ORR of Ovarian cancer=13.89% (5/36, 95% Cl 4.70-29.50).
Click to Show/Hide
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 24.00% | High Tissue factor expression (TF+++; IHC H-score=250) | ||
| Patients Enrolled |
Cervical cancer.
|
||||
| Administration Dosage |
20 mg/kg every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02001623 | Clinical Status | Phase 1/2 | ||
| Clinical Description | First-in-human, dose-escalating safety study of tissue factor specific antibody drug conjugate tisotumab vedotin (HuMax TF ADC) in patients with locally advanced and/or metastatic solid tumors known to express tissue factor. | ||||
| Primary Endpoint |
Objective response rate=24.00% (95% Cl 13.00%-37.00%).
|
||||
| Other Endpoint |
Median duration of response was 4.20 months (range: 1.00-9.70 months), comprising four patients responding for > 8 months Six-month progression-free survival was 29.00% (95% CI 17.00-43.00).
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Objective Response Rate (ORR) | 29.41% | Positive Tissue factor expression (TF+++/++; 380,000 TF receptor copy number) | ||
| Patients Enrolled |
Recurrent/metastatic cervical cancer (r/mCC).
|
||||
| Administration Dosage |
15 or 20 mg/kg once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03913741 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Open label phase 1/2 trial of tisotumab vedotin in japanese subjects with advanced solid malignancies. | ||||
| Primary Endpoint |
OrR (CR+PR)=29.41% (95% Cl, 10.30-56.00), Disease Control Rate (DCR)=70.60% (95% Cl 44.00-89.70), CR=0/17 (0%), PR=5/17 (29.41%), SD=7/17 (41.17%), PD=2/17 (11.76%).
|
||||
| Other Endpoint |
Median TTR=12 months (range, 11-27 months), median DOR=7.10 months (range, 3.10 months to not reached), median OS=11.4 months (95% Cl 6.2 -not reached) Kaplan-Meier estimates showed that the percentages of patients with an OS 6 months and 12 months were 81.60% (95% CI, 53.00-93.70) and 25.70% (95% CI, 16.00-63.90), respectively.
|
||||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04697628 | Clinical Status | Phase 3 | ||
| Clinical Description | A randomized, open-label, phase 3 trial of tisotumab vedotin vs investigator's choice chemotherapy in second- or third-line recurrent or metastatic cervical cancer. | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03657043 | Clinical Status | Phase 2 | ||
| Clinical Description | Open label phase 2 study of tisotumab vedotin for patients with platinum-resistant ovarian cancer with a safety run-in of a dose-dense regimen. | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03485209 | Clinical Status | Phase 2 | ||
| Clinical Description | Open label phase 2 study of tisotumab vedotin for locally advanced or metastatic disease in solid tumors. | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02552121 | Clinical Status | Phase 1/2 | ||
| Clinical Description | Dose-escalating and cohort expansion safety trial of tissue factor specific antibody drug conjugate tisotumab vedotin (HuMax-TF-ADC) in patients with locally advanced and/or metastatic solid tumors known to express tissue factor. | ||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 72.00% (Day 46) | High Tissue factor expression (TF+++; >300,000 TF molecules/cell) | ||
| Method Description |
TF-positive patient-derived xenograft (PDX) models were performed in athymic nude mice to evaluate the efficacy of the ADCs in vivo. Study animals were implanted unilaterally on the left flank with tumor fragments. Animals were randomized and treated as indicated in the figures. Animals were removed from study and euthanized once tumor size reached 1,200 mm3 or skin ulceration was evident. In addition, the MTV curve for the treatment group in question was no longer shown once an animal was removed from study due to size TGI and statistical analyses were conducted in the same manner as for the CDX studies. The CR and PR response definitions were as follows for the PDX studies: a PR responder had a MTV 30% of MTV at day 1 for two consecutive measurements; a CR responder had an undetectable MTV for two consecutive measurement IHC analisys: Formalin-fixed paraffin-embedded (FFPE) tissues were sectioned at 4-m thickness and mounted onto positive-charged glass slides The tissue sections were stained with the anti-TF antibody HTF-1 ADC treatment started on day 1 after animals with a tumor size of approximately 190 mm3 The model dosed weekly at 25 mg/kg for 3 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | Patient-derived xenograft (PDX) ovarian carcinomamodel | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 60) | Low Tissue factor expression (TF+; <15,000 TF molecules/cell) | ||
| Method Description |
TF-positive patient-derived xenograft (PDX) models were performed in athymic nude mice to evaluate the efficacy of the ADCs in vivo. Study animals were implanted unilaterally on the left flank with tumor fragments. Animals were randomized and treated as indicated in the figures. Animals were removed from study and euthanized once tumor size reached 1,200 mm3 or skin ulceration was evident. In addition, the MTV curve for the treatment group in question was no longer shown once an animal was removed from study due to size TGI and statistical analyses were conducted in the same manner as for the CDX studies. The CR and PR response definitions were as follows for the PDX studies: a PR responder had a MTV 30% of MTV at day 1 for two consecutive measurements; a CR responder had an undetectable MTV for two consecutive measurement IHC analisys: Formalin-fixed paraffin-embedded (FFPE) tissues were sectioned at 4-m thickness and mounted onto positive-charged glass slides The tissue sections were stained with the anti-TF antibody HTF-1 ADC treatment started on day 1 after animals with a tumor size of approximately 210 mm3 The model dosed weekly at 5 mg/kg for 2 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | Patient-derived head and neck carcinoma xenograft (PDX) model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 46) | Positive Tissue factor expression (TF+++/++; 570,000 TF receptor copy number) | ||
| Method Description |
TF-positive patient-derived xenograft (PDX) models were performed in athymic nude mice to evaluate the efficacy of the ADCs in vivo. Study animals were implanted unilaterally on the left flank with tumor fragments. Animals were randomized and treated as indicated in the figures. Animals were removed from study and euthanized once tumor size reached 1,200 mm3 or skin ulceration was evident. In addition, the MTV curve for the treatment group in question was no longer shown once an animal was removed from study due to size TGI and statistical analyses were conducted in the same manner as for the CDX studies. The CR and PR response definitions were as follows for the PDX studies: a PR responder had a MTV 30% of MTV at day 1 for two consecutive measurements; a CR responder had an undetectable MTV for two consecutive measurement IHC analisys: Formalin-fixed paraffin-embedded (FFPE) tissues were sectioned at 4-m thickness and mounted onto positive-charged glass slides The tissue sections were stained with the anti-TF antibody HTF-1 ADC treatment started on day 1 after animals with a tumor size of approximately 140 mm3 The model dosed weekly at 4 mg/kg for 3 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | Patient-derived gastric adenocarcinoma xenograft (PDX) model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 71.40% (Day 20) | High Tissue factor expression (TF+++; >300,000 TF molecules/cell) | ||
| Method Description |
Cell line-derived xenograft models were established in female SCID mice by subcutaneous injection of 5x106 (A431) tumor cells, and treatment with 3 mg/kg TF-ADCs (four injections in 2 weeks) was initiated at day 11 after tumor inoculationDetermined tumor volume after the experiment.
|
||||
| In Vivo Model | A431 cell line xenograft model | ||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 76.60% (Day 20) | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
Cell line-derived xenograft models were established in female SCID mice by subcutaneous injection of 05x106 (HCT-116) tumor cells, and treatment with 3 mg/kg TF-ADCs (four injections in 2 weeks) was initiated at day 7 after tumor inoculationDetermined tumor volume after the experiment.
|
||||
| In Vivo Model | HCT-116 cell line xenograft model | ||||
| In Vitro Model | Colon carcinoma | HCT 116 cells | CVCL_0291 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 59) | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
Cell line-derived xenograft (CDX) models The A431 epidermoid carcinoma and the HPAF-II pancreatic carcinoma cell lines were implanted subcutaneously in the flank of athymic nude mice Animals were removed from study and euthanized once tumor size reached 1200 mm3 or skin ulceration was evident ADC was dosed weekly at 5 mg/kg for 3 weeks.
|
||||
| In Vivo Model | HPAF-II xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 20) | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
Cell line-derived xenograft models were established in female SCID mice by subcutaneous injection of 2-10 x106 (HPAF-II) tumor cells, and treatment with 3 mg/kg TF-ADCs (four injections in 2 weeks) was initiated at day 13 after tumor inoculationDetermined tumor volume after the experiment.
|
||||
| In Vivo Model | HPAF-II cell line xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [8] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.00 nM
|
|||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to A431 cells, with a 72-hour incubationThis treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Erythroleukemia | HEL 92.1.7 cells (Multidrug resistance) | CVCL_2481 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
14.00 nM
|
|||
| Method Description |
A431 cells were pre-incubated for 30 min without or with 50 nM of FVIIa prior to the addition of an anti-TF ADC (Tisotumab Vedotin-tftv) titration After a 4 h incubation at 37°C, the FVIIa and ADC were washed out and the cells were cultured for another 68 h before cell viability assessment.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
14.00 nM
|
|||
| Method Description |
Cytotoxicity assay in vitroCells were seeded in 96-well plates (2,500-5,000 cells/well) and incubated for 6 hours (37°C), before adding ADCs After 3 to 5 days (37°C), the viability of the culture was assessed Staurosporine ( 10 ug/mL) was used a positive control (100% cell death) and untreated cells were used as a negative control.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [9] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
14.00 nM
|
|||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to A431 cells, with a 4-hour incubation followed by removal of excess ADC and culture for another 68 hours This treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
411.00 ng/mL
|
|||
| Method Description |
Cytotoxicity assay in vitroCells were seeded in 96-well plates (2,500-5,000 cells/well) and incubated for 6 hours (37°C), before adding ADCs After 3 to 5 days (37°C), the viability of the culture was assessed Staurosporine ( 10 ug/mL) was used a positive control (100% cell death) and untreated cells were used as a negative control.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 ug/mL | |||
| Method Description |
Cytotoxicity assay in vitroCells were seeded in 96-well plates (2,500-5,000 cells/well) and incubated for 6 hours (37°C), before adding ADCs After 3 to 5 days (37°C), the viability of the culture was assessed Staurosporine ( 10 ug/mL) was used a positive control (100% cell death) and untreated cells were used as a negative control.
|
||||
| In Vitro Model | Colon carcinoma | HCT 116 cells | CVCL_0291 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 mg/mL | |||
| Method Description |
Cytotoxicity assay in vitroCells were seeded in 96-well plates (2,500-5,000 cells/well) and incubated for 6 hours (37°C), before adding ADCs After 3 to 5 days (37°C), the viability of the culture was assessed Staurosporine ( 10 ug/mL) was used a positive control (100% cell death) and untreated cells were used as a negative control.
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | TOV-21G cells | CVCL_3613 | ||
References
