Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0OVAOS
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
SGN-CD19A
|
|||||
| Synonyms |
Anti-CD19-mafodotin; Anti-CD19-mcMMAF; Anti-CD19-vcMMAE; Denintuzumab mafodotin; SGN-19A; SGN-CD19A
Click to Show/Hide
|
|||||
| Organization |
Seagen Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
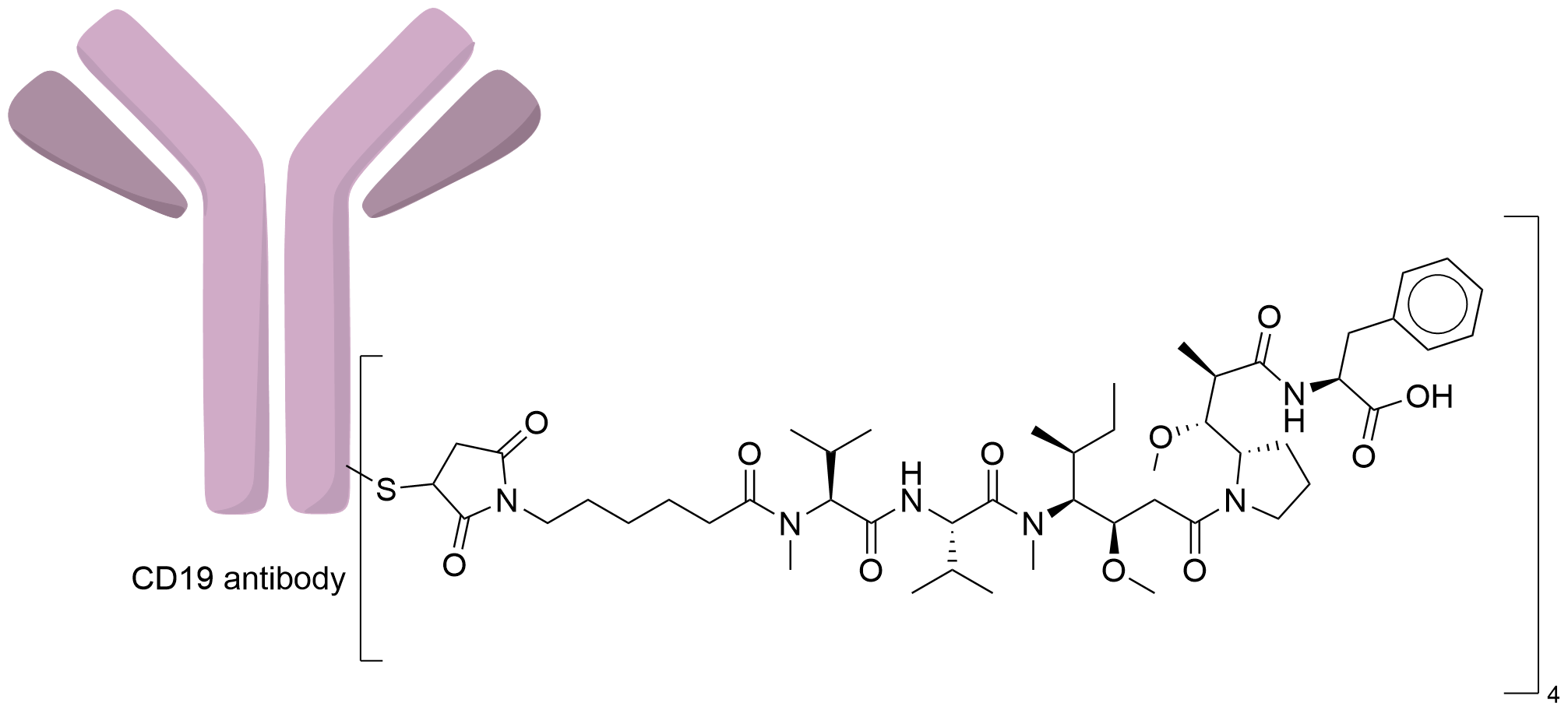
|
|||||
| Antibody Name |
Denintuzumab
|
Antibody Info | ||||
| Antigen Name |
B-lymphocyte antigen CD19 (CD19)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin F
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
mafodotin
|
|||||
| TTD ID | ||||||
