Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0OUXWT
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Rolinsatamab talirine
|
|||||
| Synonyms |
ABBV 176; ABBV-176; ABV176; DC-1630993; Rolinsatamab talirine; h16f
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 4 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
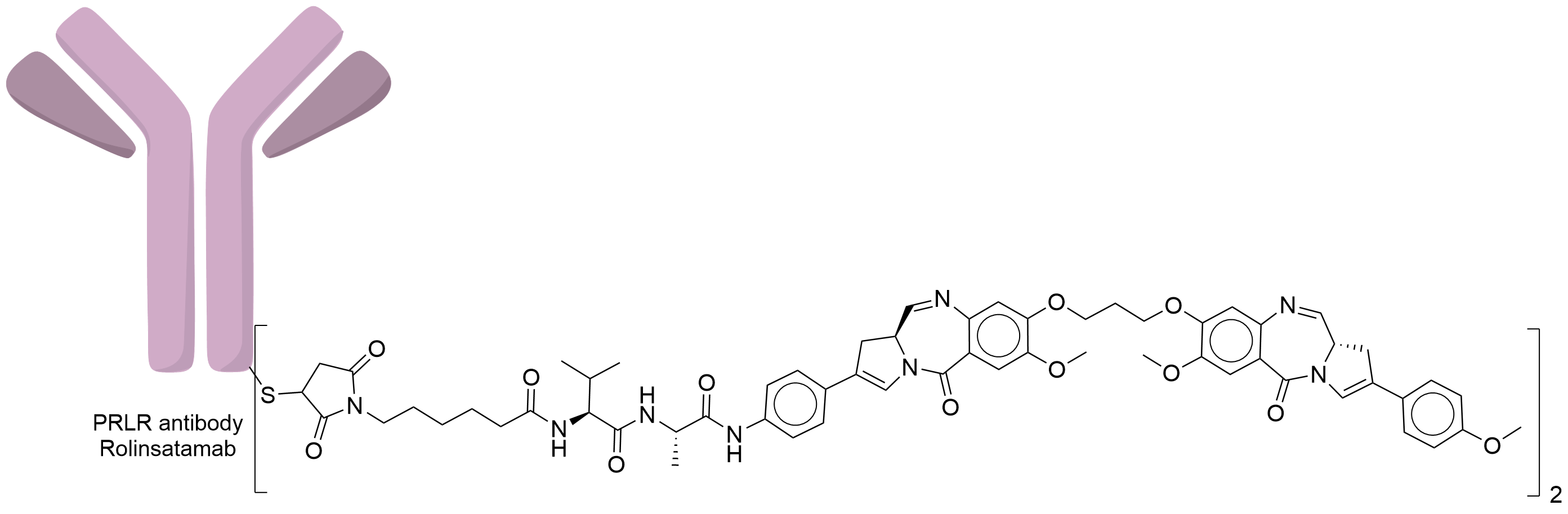
|
|||||
| Antibody Name |
Rolinsatamab
|
Antibody Info | ||||
| Antigen Name |
Prolactin receptor (PRLR)
|
Antigen Info | ||||
| Payload Name |
SGD-1882
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
Mc-Val-Ala
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
talirine
|
|||||
| Puchem SID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Locally advanced or metastatic solid tumor types associated with PRLR expression, including breast cancer, colorectal cancer, and adrenocortical carcinoma. Patients had progressed on prior treatment, were not amenable to treatment with curative intent, and had no other therapy options known to provide clinical benefit, or were ineligible for such therapies. Patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate bone marrow, renal, and hepatic function.
Click to Show/Hide
|
||||
| Administration Dosage |
Initial dose was 2.70 ug/kg, dose increment was capped at a 100% increase, or at a 50% increase if a grade2 drug-related toxicity had been observed, intravenously every 21 days.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT03145909 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study evaluating the safety, pharmacokinetics and anti-tumor activity of ABBV-176 in subjects with advanced solid tumors likely to express prolactin receptor (PRLR). | ||||
| Primary Endpoint |
MtD was not formally determined, as identification of a tolerable dose was confounded by late-onset toxicities. ABBV-176 was associated with significant toxicity in this phase 1, dose-escalation study.
|
||||
| Other Endpoint |
No patient had an objective response.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.50% | |||
| Method Description |
Mice implanted with BT-474 breast cancer model and dosed with a single dose of ABBV-176 at 0.5 mg/kg.
|
||||
| In Vivo Model | BT-474 CDX | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% | |||
| Method Description |
Mice implanted with BT-474 breast cancer model and dosed with a single dose of ABBV-176 at 0.1 mg/kg.
|
||||
| In Vivo Model | BT-474 CDX | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% | |||
| Method Description |
Mice implanted with BT-474 breast cancer model and dosed with a single dose of ABBV-176 at 0.3 mg/kg.
|
||||
| In Vivo Model | BT-474 CDX | ||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.50 pM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Breast adenocarcinoma | CAMA-1 cells | CVCL_1115 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.11 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Colon adenocarcinoma | SW403 cells | CVCL_0545 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.16 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Ovarian clear cell adenocarcinoma | SMOV-2 cells | CVCL_S920 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.24 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.26 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.32 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.60 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Endometrial adenocarcinoma | AN3-CA cells | CVCL_0028 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.77 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.20 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Adult hepatocellular carcinoma | Huh-7 cells | CVCL_0336 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.60 nM
|
|||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Hepatoblastoma | Hep-G2 cells | CVCL_0027 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 22.00 nM | |||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Invasive breast carcinoma of no special type | UACC-812 cells | CVCL_1781 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 22.00 nM | |||
| Method Description |
The inhibitory activity of ABBV-176 against cancer cell growth was evaluated in various human cancer cell lines in vitro. The cells were treated 144 hours.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
References
