Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0OMVBK
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Azintuxizumab vedotin
|
|||||
| Synonyms |
ABBV 838; ABBV-838; ABBV838; Azintuxizumab vedotin
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
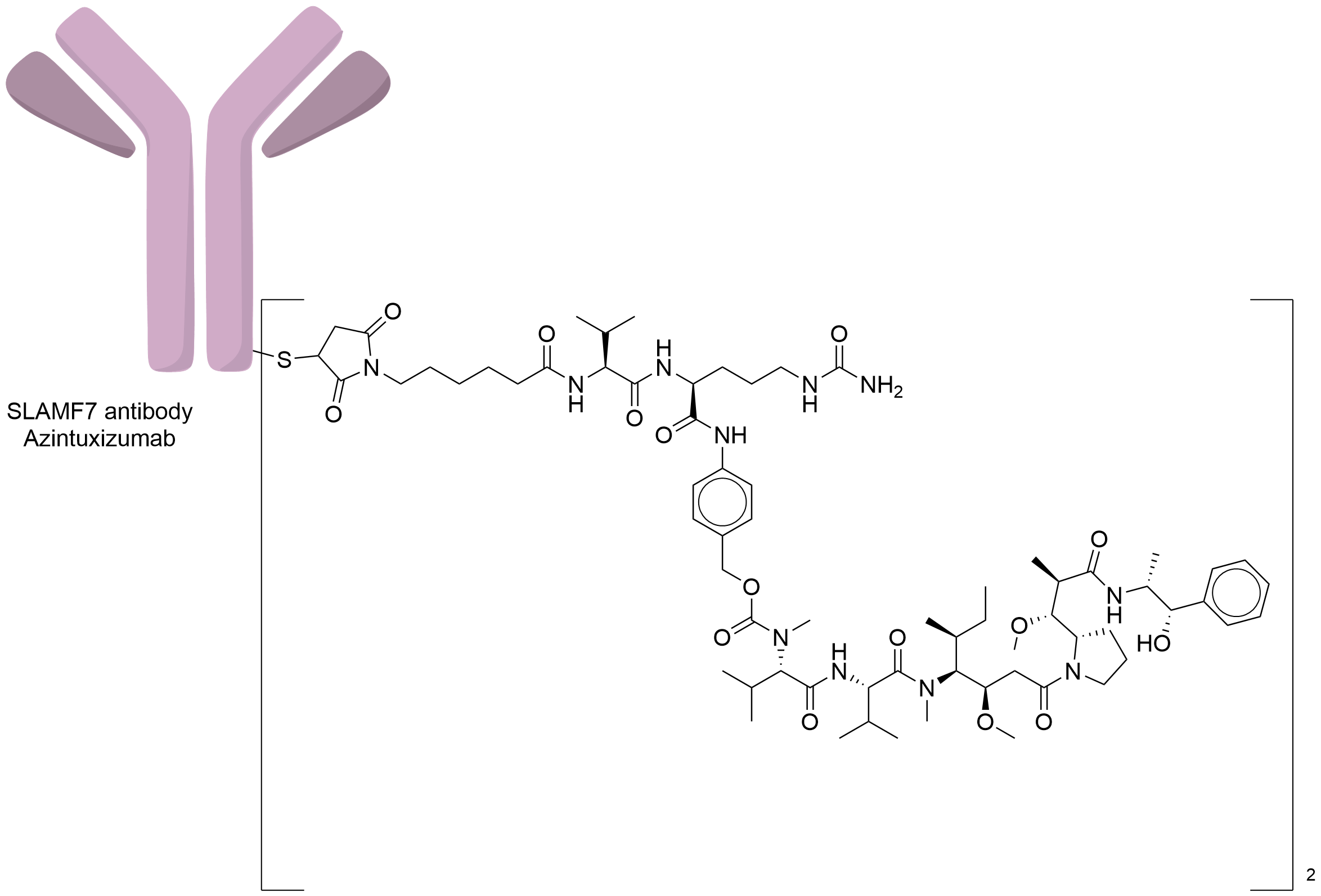
|
|||||
| Antibody Name |
Azintuxizumab
|
Antibody Info | ||||
| Antigen Name |
SLAM family member 7 (SLAMF7)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
vedotin
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
10.67%
|
|||
| Patients Enrolled |
Relapsed or refractory multiple myeloma (RRMM) and Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; were not eligible for stem cell/bone marrow transplant or had refused stem cell/bone marrow transplant, or had relapsed after autologous or allogeneic stem cell/bone marrow transplant.
|
||||
| Administration Dosage |
ABBV-838 (3+3 design) intravenously starting from 0.60 mg/kg up to 6.00 mg/kg for 3-week dosing intervals (Q3W). Patients could continue ABBV-838 for up to 24 months. Assessment of alternate dosing intervals (Q1W and Q2W) was conducted in parallel.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02462525 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-838, an antibody drug conjugate, in subjects with relapsed and refractory multiple myeloma. | ||||
| Primary Endpoint |
OrR=10.67% (N=8/75, 95% Cl 4.7-19.9), very good partial response (VGPR)=2.67% (N=2), PR=8.00% (N=6). Median DOR=4 months.
|
||||
| Other Endpoint |
The MTD was not reached. The selected recommended dose for the expansion cohort was 5.00 mg/kg Q3W.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02951117 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1b, open label, multicenter, dose escalation study of venetoclax and ABBV-838 combination therapy with dexamethasone in subjects with relapsed or refractory multiple myeloma. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02462525 | Clinical Status | Phase 1 | ||
| Clinical Description | A multicenter, phase 1/1b, open-label, dose-escalation study of ABBV-838, an antibody drug conjugate, in subjects with relapsed and refractory multiple myeloma. | ||||
References
