Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0OBHVE
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
54E-Val-Cit-MMAE
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3-4
|
|||||
| Structure |
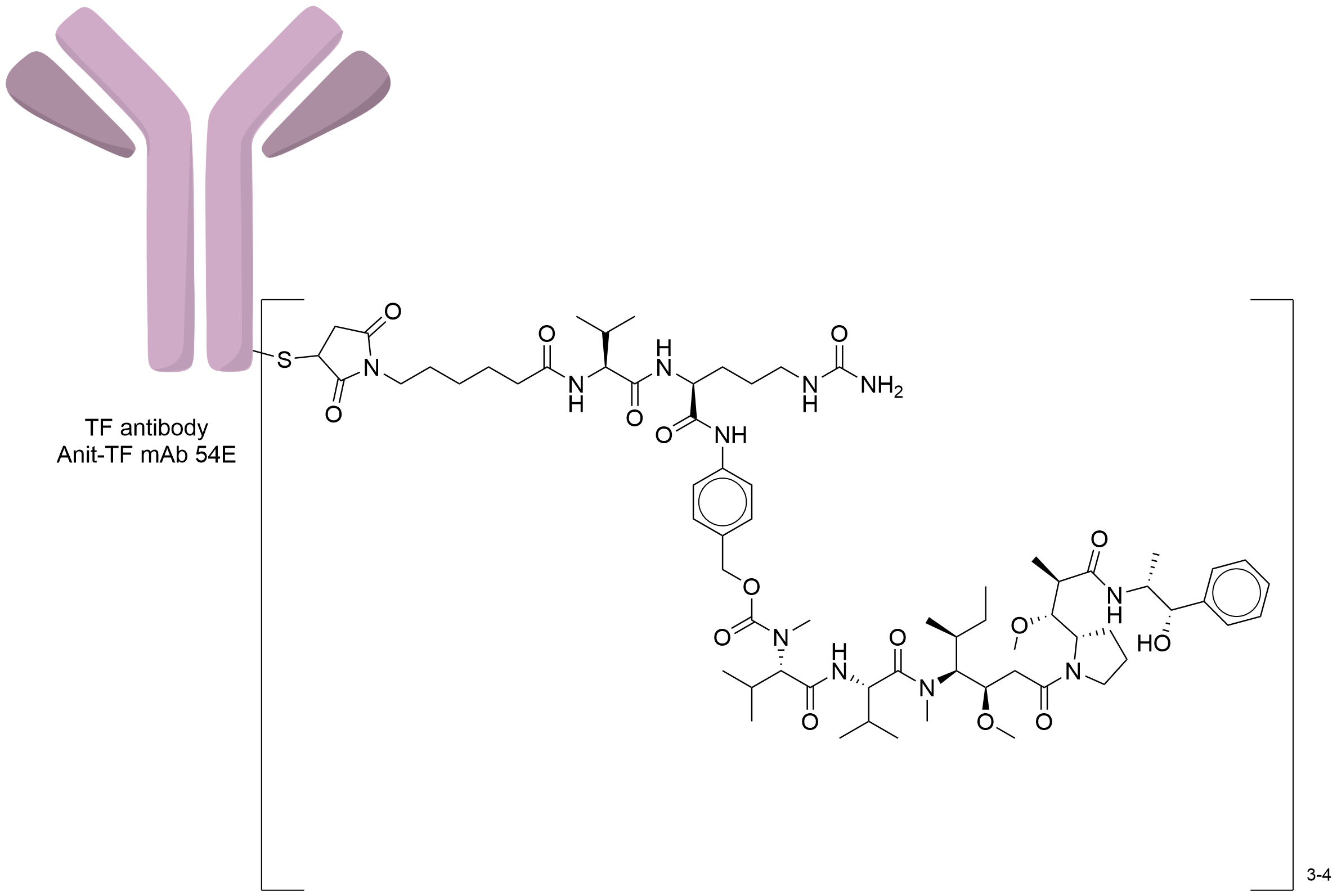
|
|||||
| Antibody Name |
Anit-TF mAb 54E
|
Antibody Info | ||||
| Antigen Name |
Tissue factor (F3)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Effective Concentration (EC50) |
3
|
nM
|
MDA-MB-231 cells
|
Breast adenocarcinoma
|
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 3.00 nM | Positive Tissue factor expression (TF+++/++; 320,000 TF receptor copy number) | ||
| Method Description |
Antibody-dependent cellular cytotoxicity (ADCC) A431 cells were plated on a microtiter plate The following day, the cells were incubated with a ten-point 1:3 dilution titration of anti-TF antibodies or the ADCs starting at 50 nM An ADCC effector-to-target cell ratio of 8:1 was added to each well and incubated for 6 h at 37°C Luciferase Assay Reagent was added to each well to measure luminescence on an Envision plate reader Antibody-dependent cellular cytotoxicity (ADCC) reporter luminescence was evaluated after a 6-hour incubation of the reporter Jurkat cell line with TF-positive A431 cells and a titration of anti-TF antibody or ADC The ADCC reporter luminescence EC50 values for each anti-TF antibody or ADC are listed.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 14.00 nM | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
A431 cells were pre-incubated for 30 min without or with 50 nM of FVIIa prior to the addition of an anti-TF ADC (54E-vc-MMAE) titration After a 4 h incubation at 37°C, the FVIIa and ADC were washed out and the cells were cultured for another 68 h before cell viability assessment.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 14.00 nM | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to A431 cells, with a 72-hour incubationThis treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 14.00 nM | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to A431 cells, with a 4-hour incubation followed by removal of excess ADC and culture for another 68 hours This treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
References
