Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0MPDIB
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ABBV-154
|
|||||
| Synonyms |
ABBV 154; ABBV-154; ABBV154
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Phase 2 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
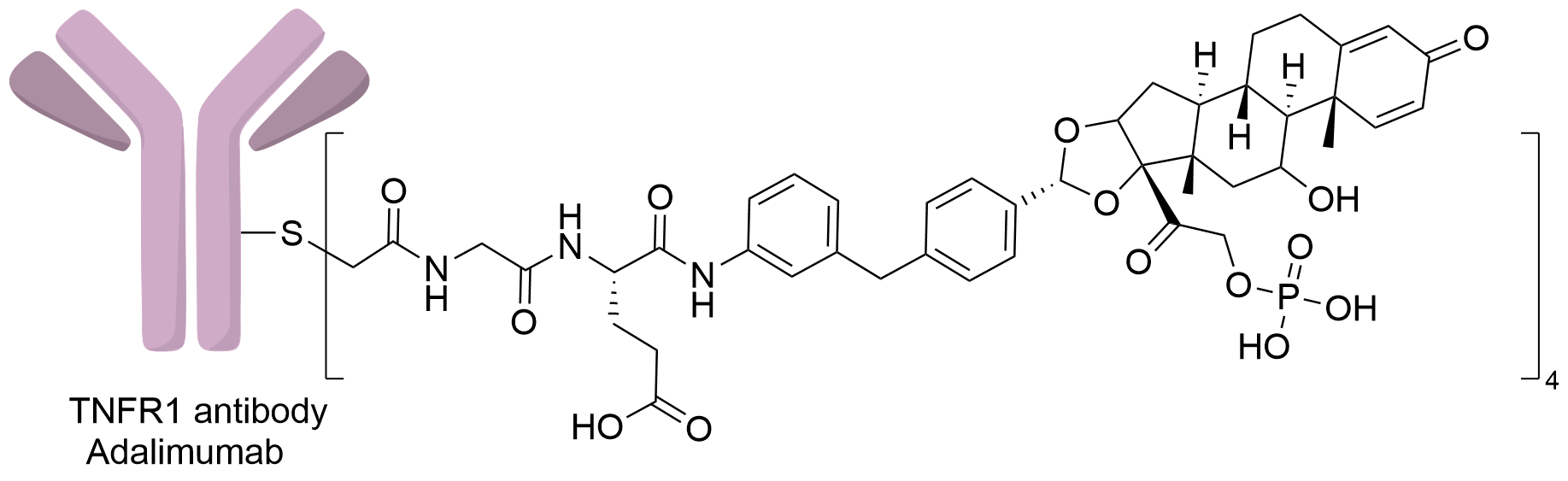
|
|||||
| Antibody Name |
Adalimumab
|
Antibody Info | ||||
| Antigen Name |
Tumor necrosis factor (TNF)
|
Antigen Info | ||||
| Payload Name |
Glucocorticoid receptor modulator
|
Payload Info | ||||
| Therapeutic Target |
Glucocorticoid receptor (NR3C1)
|
Target Info | ||||
| Linker Name |
Formyl-Gly-Glu
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05068284 | Clinical Status | Phase 2 | ||
| Clinical Description | A randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of ABBV-154 in subjects with moderately to severely active crohn's disease (CD): aim-cd. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04972968 | Clinical Status | Phase 2 | ||
| Clinical Description | A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the safety and efficacy of ABBV-154 in subjects with polymyalgia rheumatica (PMR) dependent on glucocorticoid treatment. | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04888585 | Clinical Status | Phase 2 | ||
| Clinical Description | A randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of ABBV-154 in subjects with moderately to severely active rheumatoid arthritis with inadequate response to biologic and/or targeted synthetic disease-modifying anti-rheumatic drugs (b/tsdmards). | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05556226 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1 study in healthy subjects to evaluate the relative bioavailability of ABBV-154. | ||||
References
