Linker Information
General Information of This Linker
| Linker ID |
LIN0OUTRX
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Formyl-Gly-Glu
|
|||||
| Linker Type |
Flexible reactive (thiol) linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
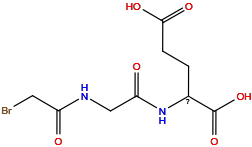
|
|||||
| Formula |
C9H13BrN2O6
|
|||||
| Isosmiles |
O=C(O)CCC(NC(=O)CNC(=O)CBr)C(=O)O
|
|||||
| InChI |
InChI=1S/C9H13BrN2O6/c10-3-6(13)11-4-7(14)12-5(9(17)18)1-2-8(15)16/h5H,1-4H2,(H,11,13)(H,12,14)(H,15,16)(H,17,18)
|
|||||
| InChIKey |
LLODMEXPRFSSOV-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
325.115
|
Polar area
|
132.8
|
||
|
Complexity
|
323.9956982
|
xlogp Value
|
-1.0683
|
|||
|
Heavy Count
|
18
|
Rot Bonds
|
8
|
|||
|
Hbond acc
|
4
|
Hbond Donor
|
4
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
ABBV-154 [Phase 2 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05068284 | Clinical Status | Phase 2 | ||
| Clinical Description |
A randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of ABBV-154 in subjects with moderately to severely active crohn's disease (CD): aim-cd.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04972968 | Clinical Status | Phase 2 | ||
| Clinical Description |
A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the safety and efficacy of ABBV-154 in subjects with polymyalgia rheumatica (PMR) dependent on glucocorticoid treatment.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04888585 | Clinical Status | Phase 2 | ||
| Clinical Description |
A randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of ABBV-154 in subjects with moderately to severely active rheumatoid arthritis with inadequate response to biologic and/or targeted synthetic disease-modifying anti-rheumatic drugs (b/tsdmards).
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05556226 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 study in healthy subjects to evaluate the relative bioavailability of ABBV-154.
|
||||
References
