Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0MEWVN
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
25A-Val-Cit-MMAE
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
3-4
|
|||||
| Structure |
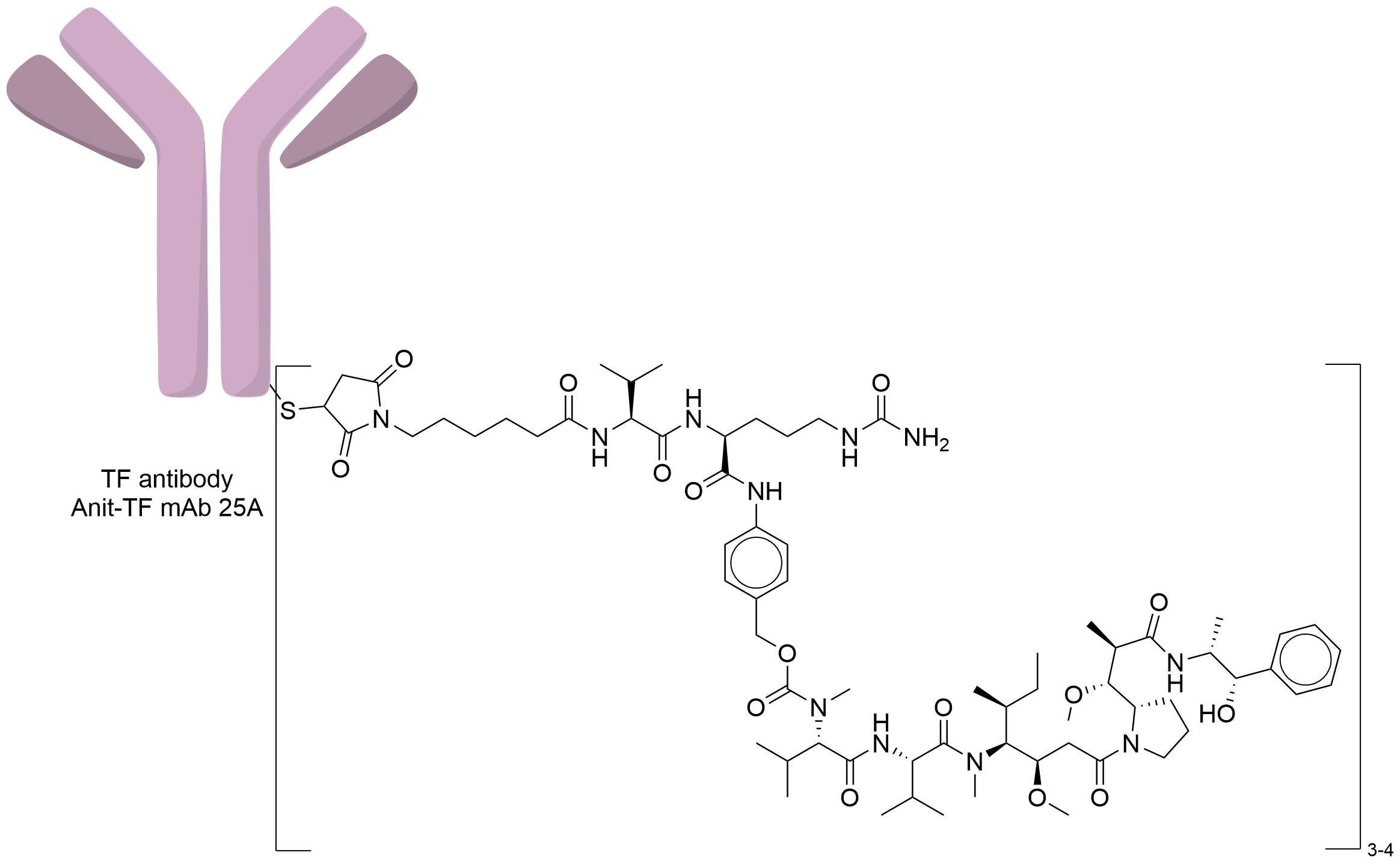
|
|||||
| Antibody Name |
Anit-TF mAb 25A
|
Antibody Info | ||||
| Antigen Name |
Tissue factor (F3)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin E
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random conjugation through reduced inter-chain cysteines.
|
|||||
| Combination Type |
Vedotin
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Patient-derived Xenograft Model
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 71.00% (Day 46) | Moderate Tissue factor expression (TF++; IHC H-score=155) | ||
| Method Description |
TF-positive patient-derived xenograft (PDX) models were performed in athymic nude mice to evaluate the efficacy of the ADCs in vivo. Study animals were implanted unilaterally on the left flank with tumor fragments. Animals were randomized and treated as indicated in the figures. Animals were removed from study and euthanized once tumor size reached 1,200 mm3 or skin ulceration was evident. In addition, the MTV curve for the treatment group in question was no longer shown once an animal was removed from study due to size TGI and statistical analyses were conducted in the same manner as for the CDX studies. The CR and PR response definitions were as follows for the PDX studies: a PR responder had a MTV 30% of MTV at day 1 for two consecutive measurements; a CR responder had an undetectable MTV for two consecutive measurement IHC analisys: Formalin-fixed paraffin-embedded (FFPE) tissues were sectioned at 4-m thickness and mounted onto positive-charged glass slides The tissue sections were stained with the anti-TF antibody HTF-1 ADC treatment started on day 1 after animals with a tumor size of approximately 190 mm3 The model dosed weekly at 25 mg/kg for 3 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | Patient-derived xenograft (PDX) ovarian carcinomamodel | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 60) | Positive Tissue factor expression (TF+++/++; 320,000 TF receptor copy number) | ||
| Method Description |
TF-positive patient-derived xenograft (PDX) models were performed in athymic nude mice to evaluate the efficacy of the ADCs in vivo. Study animals were implanted unilaterally on the left flank with tumor fragments. Animals were randomized and treated as indicated in the figures. Animals were removed from study and euthanized once tumor size reached 1,200 mm3 or skin ulceration was evident. In addition, the MTV curve for the treatment group in question was no longer shown once an animal was removed from study due to size TGI and statistical analyses were conducted in the same manner as for the CDX studies. The CR and PR response definitions were as follows for the PDX studies: a PR responder had a MTV 30% of MTV at day 1 for two consecutive measurements; a CR responder had an undetectable MTV for two consecutive measurement IHC analisys: Formalin-fixed paraffin-embedded (FFPE) tissues were sectioned at 4-m thickness and mounted onto positive-charged glass slides The tissue sections were stained with the anti-TF antibody HTF-1 ADC treatment started on day 1 after animals with a tumor size of approximately 210 mm3 The model dosed weekly at 5 mg/kg for 2 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | Patient-derived head and neck carcinoma xenograft (PDX) model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 46) | Positive Tissue factor expression (TF+++/++; 320,000 TF receptor copy number) | ||
| Method Description |
TF-positive patient-derived xenograft (PDX) models were performed in athymic nude mice to evaluate the efficacy of the ADCs in vivo. Study animals were implanted unilaterally on the left flank with tumor fragments. Animals were randomized and treated as indicated in the figures. Animals were removed from study and euthanized once tumor size reached 1,200 mm3 or skin ulceration was evident. In addition, the MTV curve for the treatment group in question was no longer shown once an animal was removed from study due to size TGI and statistical analyses were conducted in the same manner as for the CDX studies. The CR and PR response definitions were as follows for the PDX studies: a PR responder had a MTV 30% of MTV at day 1 for two consecutive measurements; a CR responder had an undetectable MTV for two consecutive measurement IHC analisys: Formalin-fixed paraffin-embedded (FFPE) tissues were sectioned at 4-m thickness and mounted onto positive-charged glass slides The tissue sections were stained with the anti-TF antibody HTF-1 ADC treatment started on day 1 after animals with a tumor size of approximately 140 mm3 The model dosed weekly at 4 mg/kg for 3 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | Patient-derived gastric adenocarcinoma xenograft (PDX) model | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 67.00% (Day 49) | Positive Tissue factor expression (TF+++/++; 570,000 TF receptor copy number) | ||
| Method Description |
Cell line-derived xenograft (CDX) models MDA-MB-231 epidermoid carcinoma and the HPAF-II pancreatic carcinoma cell lines were implanted subcutaneously in the flank of athymic nude mice Animals were removed from study and euthanized once tumor size reached 1200 mm3 or skin ulceration was evident ADC was dosed weekly at 2 mg/kg for 2 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | MDA-MB-231 cell line xenograft model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 98.00% (Day 49) | Positive Tissue factor expression (TF+++/++; 570,000 TF receptor copy number) | ||
| Method Description |
Cell line-derived xenograft (CDX) models MDA-MB-231 epidermoid carcinoma and the HPAF-II pancreatic carcinoma cell lines were implanted subcutaneously in the flank of athymic nude mice Animals were removed from study and euthanized once tumor size reached 1200 mm3 or skin ulceration was evident ADC was dosed weekly at 4 mg/kg for 2 weeks.
Click to Show/Hide
|
||||
| In Vivo Model | MDA-MB-231 cell line xenograft model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 59) | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
Cell line-derived xenograft (CDX) models The A431 epidermoid carcinoma and the HPAF-II pancreatic carcinoma cell lines were implanted subcutaneously in the flank of athymic nude mice Animals were removed from study and euthanized once tumor size reached 1200 mm3 or skin ulceration was evident ADC was dosed weekly at 5 mg/kg for 3 weeks.
|
||||
| In Vivo Model | HPAF-II xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 100.00% (Day 39) | Low FOLR1 expression (FOLR1+) | ||
| Method Description |
Cell line-derived xenograft (CDX) models The A431 epidermoid carcinoma and the HPAF-II pancreatic carcinoma cell lines were implanted subcutaneously in the flank of athymic nude mice Animals were removed from study and euthanized once tumor size reached 1200 mm3 or skin ulceration was evident ADC was dosed weekly at 2 mg/kg for 2 weeks.
|
||||
| In Vivo Model | HPAF-II xenograft model | ||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 26.00 nM | Positive Tissue factor expression (TF+++/++; 320,000 TF receptor copy number) | ||
| Method Description |
Antibody-dependent cellular cytotoxicity (ADCC) A431 cells were plated on a microtiter plate The following day, the cells were incubated with a ten-point 1:3 dilution titration of anti-TF antibodies or the ADCs starting at 50 nM An ADCC effector-to-target cell ratio of 8:1 was added to each well and incubated for 6 h at 37°C Luciferase Assay Reagent was added to each well to measure luminescence on an Envision plate reader Antibody-dependent cellular cytotoxicity (ADCC) reporter luminescence was evaluated after a 6-hour incubation of the reporter Jurkat cell line with TF-positive A431 cells and a titration of anti-TF antibody or ADC The ADCC reporter luminescence EC50 values for each anti-TF antibody or ADC are listed.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 6.00 nM | High Tissue factor expression (TF+++; IHC H-score=250) | ||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to HPAF-II cells, with a 72-hour incubationThis treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | HPAF-II cells | CVCL_0313 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 14.00 nM | Positive Tissue factor expression (TF+++/++; 570,000 TF receptor copy number) | ||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to 5-day old cultures of MDA-MB-231 cells, with a 72-hour incubationThis treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 18.00 nM | High Tissue factor expression (TF+++; IHC H-score=250) | ||
| Method Description |
A431 cells were pre-incubated for 30 min without or with 50 nM of FVIIa prior to the addition of an anti-TF ADC (25A-vc-MMAE) titration After a 4 h incubation at 37°C, the FVIIa and ADC were washed out and the cells were cultured for another 68 h before cell viability assessment.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 14.00 nM | Positive Tissue factor expression (TF+++/++; 380,000 TF receptor copy number) | ||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to A431 cells, with a 72-hour incubationThis treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 14.00 nM | Positive Tissue factor expression (TF+++/++; 320,000 TF receptor copy number) | ||
| Method Description |
To evaluate ADC cytotoxicity, cells were plated in 384-well plates Anti-TF antibodies conjugated to MC-vc-PAB-MMAE were serially diluted as shown Plates were incubated for 3 days, followed by lysis in CTG assay reagent For each ADC, the IC50 and its associated 95% confidence interval (95% CI) were calculated Titrations of the TF-specific ADCs were added to A431 cells, with a 4-hour incubation followed by removal of excess ADC and culture for another 68 hours This treatment resulted in efficacious cell killing.
Click to Show/Hide
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
References
