Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0LODVW
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
MEDI-547
|
|||||
| Synonyms |
MEDI 547; MEDI-547; MEDI547
Click to Show/Hide
|
|||||
| Organization |
MedImmune LLC; AstraZeneca PLC
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
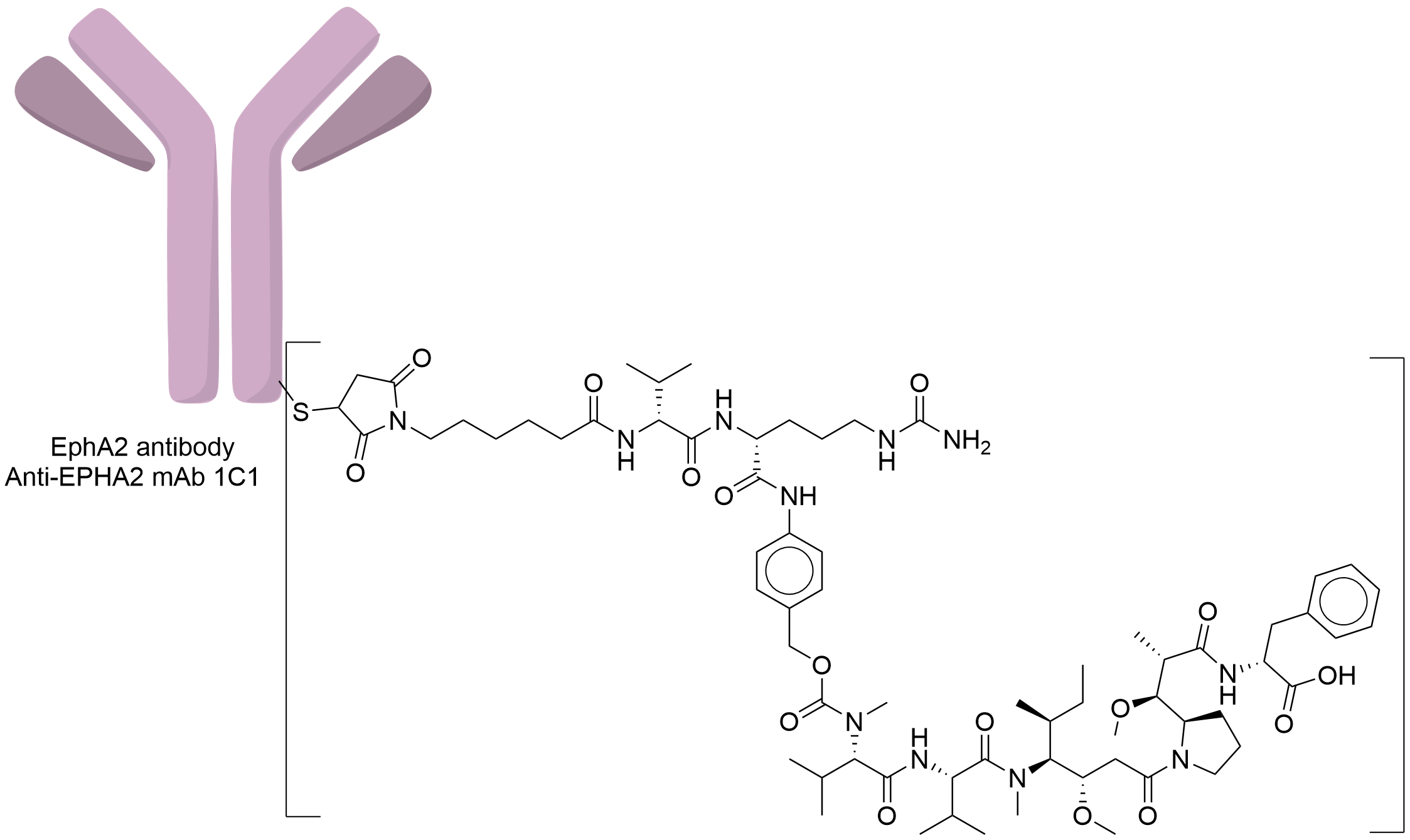
|
|||||
| Antibody Name |
Anti-EPHA2 mAb 1C1
|
Antibody Info | ||||
| Antigen Name |
Ephrin type-A receptor 2 (EPHA2)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin F
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
mafodotin
|
|||||
| Puchem SID | ||||||
| DrugMap ID | ||||||
| TTD ID | ||||||
| ChEBI ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
0.00%
|
|||
| Patients Enrolled |
Malignant solid tumor thought to be associated with increased expression of EphA2 (endometrial, breast, ovarian, prostate, non-small cell lung, colon, esophageal, gastric, and bladder cancers, renal cell carcinoma, melanoma), relapsed or refractory to standard therapy, and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0-2.
Click to Show/Hide
|
||||
| Administration Dosage |
0.08 mg/kg, 1-h intravenous (IV) infusion once q3wks or qwk for 3 consecutive weeks until unacceptable toxicity, progressive disease.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT00796055 | Clinical Status | Phase 1 | ||
| Clinical Description | A phase 1, open-label study of MEDI-547 to evaluate the safety, tolerability, pharmacokinetics, and biologic activity of intravenous administration in subjects with relapsed or refractory solid tumors associated with epha2 expression. | ||||
| Primary Endpoint |
Best response included progressive disease (n=5, 83.33%) and stable disease (n=1, 16.67%), No complete or partial tumor responses.
|
||||
| Other Endpoint |
MTD could not be selected.
|
||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.00% | Negative EPHA2 expression (EPHA2-) | ||
| Method Description |
Mice injected with EphA2-negative SPEC-2 cell was assigned to one of four groups (n = 10 mice per group),MEDI-547,3 mg/kg weekly.
|
||||
| In Vivo Model | Endometrial cancer CDX model | ||||
| In Vitro Model | Endometrial cancer | Endometrial cancer cells | Homo sapiens | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 86.67% | Positive EPHA2 expression (EPHA2+++/++) | ||
| Method Description |
Mice injected with either Hec-1A or Ishikawa were assigned to one of four groups (n = 10 mice per group),MEDI-547,3 mg/kg weekly.
|
||||
| In Vivo Model | Endometrial cancer CDX model | ||||
| In Vitro Model | Endometrial adenocarcinoma | HEC-1-A cells | CVCL_0293 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.00% | Positive EPHA2 expression (EPHA2+++/++) | ||
| Method Description |
Mice injected with either Hec-1A or Ishikawa were assigned to one of four groups (n = 10 mice per group),MEDI-547,3 mg/kg weekly.
|
||||
| In Vivo Model | Endometrial cancer CDX model | ||||
| In Vitro Model | Endometrial adenocarcinoma | Ishikawa cells | CVCL_2529 | ||
References
